Reduced inhibition of cortical glutamate and GABA release by halothane in mice lacking the K+ channel, TREK-1
- PMID: 17828284
- PMCID: PMC2078222
- DOI: 10.1038/sj.bjp.0707450
Reduced inhibition of cortical glutamate and GABA release by halothane in mice lacking the K+ channel, TREK-1
Abstract
Background and purpose: Deletion of TREK-1, a two-pore domain K(+) channel (K(2P)) activated by volatile anaesthetics, reduces volatile anaesthetic potency in mice, consistent with a role for TREK-1 as an anaesthetic target. We used TREK-1 knockout mice to examine the presynaptic function of TREK-1 in transmitter release and its role in the selective inhibition of glutamate vs GABA release by volatile anaesthetics.
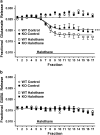
Experimental approach: The effects of halothane on 4-aminopyridine-evoked and basal [(3)H]glutamate and [(14)C]GABA release from cerebrocortical nerve terminals isolated from TREK-1 knockout (KO) and littermate wild-type (WT) mice were compared. TREK-1 was quantified by immunoblotting of nerve terminal preparations.
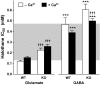
Key results: Deletion of TREK-1 significantly reduced the potency of halothane inhibition of 4-aminopyridine-evoked release of both glutamate and GABA without affecting control evoked release or the selective inhibition of glutamate vs GABA release. TREK-1 deletion also reduced halothane inhibition of basal glutamate release, but did not affect basal GABA release.
Conclusions and implications: The reduced sensitivity of glutamate and GABA release to inhibition by halothane in TREK-1 KO nerve terminals correlates with the reduced anaesthetic potency of halothane in TREK-1 KO mice observed in vivo. A presynaptic role for TREK-1 was supported by the enrichment of TREK-1 in isolated nerve terminals determined by immunoblotting. This study represents the first evidence for a link between an anaesthetic-sensitive 2-pore domain K(+) channel and presynaptic function, and provides further support for presynaptic mechanisms in determining volatile anaesthetic action.
Figures
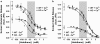


Similar articles
-
Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: 4-aminopyridine-evoked release.J Pharmacol Exp Ther. 2006 Jan;316(1):216-23. doi: 10.1124/jpet.105.090662. Epub 2005 Sep 20. J Pharmacol Exp Ther. 2006. PMID: 16174800
-
Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: basal release.J Pharmacol Exp Ther. 2006 Jan;316(1):208-15. doi: 10.1124/jpet.105.090647. Epub 2005 Sep 20. J Pharmacol Exp Ther. 2006. PMID: 16174801
-
The effects of general anesthetics on norepinephrine release from isolated rat cortical nerve terminals.Anesth Analg. 2002 Nov;95(5):1274-81, table of contents. doi: 10.1097/00000539-200211000-00032. Anesth Analg. 2002. PMID: 12401610
-
The TREK K2P channels and their role in general anaesthesia and neuroprotection.Trends Pharmacol Sci. 2004 Nov;25(11):601-8. doi: 10.1016/j.tips.2004.09.003. Trends Pharmacol Sci. 2004. PMID: 15491783 Review.
-
Functional significance of co-localization of GABA and Glu in nerve terminals: a hypothesis.Curr Top Med Chem. 2006;6(10):969-73. doi: 10.2174/156802606777323737. Curr Top Med Chem. 2006. PMID: 16787271 Review.
Cited by
-
Isoflurane inhibits the neurotransmitter release machinery.J Neurophysiol. 2009 Aug;102(2):1265-73. doi: 10.1152/jn.00252.2009. Epub 2009 Jun 10. J Neurophysiol. 2009. PMID: 19515956 Free PMC article.
-
Potentiation of Schaffer-Collateral CA1 Synaptic Transmission by eEF2K and p38 MAPK Mediated Mechanisms.Front Cell Neurosci. 2016 Oct 25;10:247. doi: 10.3389/fncel.2016.00247. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27826228 Free PMC article.
-
Two-pore domain potassium channels enable action potential generation in the absence of voltage-gated potassium channels.Pflugers Arch. 2015 May;467(5):989-99. doi: 10.1007/s00424-014-1660-6. Epub 2014 Dec 9. Pflugers Arch. 2015. PMID: 25482670 Free PMC article. Review.
-
Nicotinic receptor-evoked hippocampal norepinephrine release is highly sensitive to inhibition by isoflurane.Br J Anaesth. 2009 Mar;102(3):355-60. doi: 10.1093/bja/aen387. Epub 2009 Feb 2. Br J Anaesth. 2009. PMID: 19189985 Free PMC article.
-
Interactions between Glycine and Glutamate through Activation of Their Transporters in Hippocampal Nerve Terminals.Biomedicines. 2023 Nov 27;11(12):3152. doi: 10.3390/biomedicines11123152. Biomedicines. 2023. PMID: 38137373 Free PMC article.
References
-
- Bai X, Bugg GJ, Greenwood SL, Glazier JD, Sibley CP, Baker PN, et al. Expression of TASK and TREK, two-pore domain K+ channels, in human myometrium. Reproduction. 2005;129:525–530. - PubMed
-
- Franks NP, Honore E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol Sci. 2004;25:601–608. - PubMed
-
- Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two-pore domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol. 2004;65:443–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

