Silymarin prevents palmitate-induced lipotoxicity in HepG2 cells: involvement of maintenance of Akt kinase activation
- PMID: 17845508
- PMCID: PMC4219607
- DOI: 10.1111/j.1742-7843.2007.00116.x
Silymarin prevents palmitate-induced lipotoxicity in HepG2 cells: involvement of maintenance of Akt kinase activation
Abstract
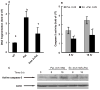
Whereas adipocytes have a unique capacity to store excess free fatty acids in the form of triglyceride in lipid droplets, non-adipose tissues, such as liver, have a limited capacity for storage of lipids. Saturated long-chain fatty acids, such as palmitate, are the major contributors to lipotoxicity. Silymarin is a mixture of flavonolignans, extracted from the milk thistle (Silibum marianum). Its hepatoprotective properties have been studied both in vitro and in vivo; however, its effect on palmitate-induced lipotoxicity has not been investigated. The objective of this study was to investigate (i) whether silymarin could protect HepG2 cells from palmitate-induced cell death in an in vitro model, and (ii) possible mechanisms involved in this hepatoprotective role of silymarin. HepG2 cells were treated with palmitate in the absence or presence of silymarin and supernatants or cell lysates were collected at varying time-points. Cell death was assayed by measuring DNA fragmentation, caspase-3 activity and lactate dehydrogenase release. Lipid peroxidation was assessed by measuring malondialdehyde and 4-hydroxyalkenals. Akt kinase activity was also measured. Incubation with palmitate caused significant death in HepG2 cells. Palmitate incubation did not cause significant changes in reactive oxygen species production or intracellular glutathione content, but markedly inhibited Akt kinase activity. Pre-treatment of HepG2 cells with silymarin prevented palmitate-induced inhibition of Akt kinase activity and attenuated cell death. Our results suggest that silymarin may be an effective agent in protecting hepatocytes from saturated fatty acids-induced cell death. These data also provide a further rationale for exploration of the use of silymarin in the treatment of non-alcoholic steatohepatitis.
Figures




References
-
- Harrison SA, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis. 2004;8:861–79. - PubMed
-
- Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. - PubMed
-
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–5. - PubMed
-
- Choudhury J, Sanyal AJ. Insulin resistance in NASH. Front Biosci. 2005;10:1520–33. - PubMed
-
- Choudhury J, Sanyal AJ. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:575–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

