Alternative splicing of TGF-betas and their high-affinity receptors T beta RI, T beta RII and T beta RIII (betaglycan) reveal new variants in human prostatic cells
- PMID: 17845732
- PMCID: PMC2075524
- DOI: 10.1186/1471-2164-8-318
Alternative splicing of TGF-betas and their high-affinity receptors T beta RI, T beta RII and T beta RIII (betaglycan) reveal new variants in human prostatic cells
Abstract
Background: The transforming growth factors (TGF)-beta, TGF-beta1, TGF-beta2 and TGF-beta 3, and their receptors [T beta RI, T beta RII, T beta R III (betaglycan)] elicit pleiotropic functions in the prostate. Although expression of the ligands and receptors have been investigated, the splice variants have never been analyzed. We therefore have analyzed all ligands, the receptors and the splice variants T beta RIB, T beta RIIB and TGF-beta 2B in human prostatic cells.
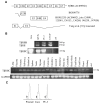
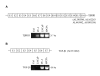
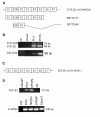
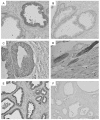
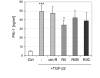
Results: Interestingly, a novel human receptor transcript T beta RIIC was identified, encoding additional 36 amino acids in the extracellular domain, that is expressed in the prostatic cancer cells PC-3, stromal hPCPs, and other human tissues. Furthermore, the receptor variant T beta RIB with four additional amino acids was identified also in human. Expression of the variant T beta RIIB was found in all prostate cell lines studied with a preferential localization in epithelial cells in some human prostatic glands. Similarly, we observed localization of T beta RIIC and TGF-beta 2B mainly in the epithelial cells with a preferential localization of TGF-beta 2B in the apical cell compartment. Whereas in the androgen-independent hPCPs and PC-3 cells all TGF-beta ligands and receptors are expressed, the androgen-dependent LNCaP cells failed to express all ligands. Additionally, stimulation of PC-3 cells with TGF-beta2 resulted in a significant and strong increase in secretion of plasminogen activator inhibitor-1 (PAI-1) with a major participation of T beta RII.
Conclusion: In general, expression of the splice variants was more heterogeneous in contrast to the well-known isoforms. The identification of the splice variants T beta RIB and the novel isoform T beta RIIC in man clearly contributes to the growing complexity of the TGF-beta family.
Figures


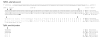

Similar articles
-
Analysis of the mRNA expression of the TGF-Beta family in testicular cells and localization of the splice variant TGF-beta2B in testis.Mol Reprod Dev. 2006 Oct;73(10):1211-20. doi: 10.1002/mrd.20399. Mol Reprod Dev. 2006. PMID: 16868931
-
Transforming growth factor-beta receptors in human cancer cell lines: analysis of transcript, protein and proliferation.Anticancer Res. 1997 May-Jun;17(3C):1849-60. Anticancer Res. 1997. PMID: 9216635
-
Differential expression of transforming growth factor beta receptors in human pancreatic adenocarcinoma.Anticancer Res. 2000 Jan-Feb;20(1A):43-51. Anticancer Res. 2000. PMID: 10769633
-
Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart.Cells Tissues Organs. 2007;185(1-3):146-56. doi: 10.1159/000101315. Cells Tissues Organs. 2007. PMID: 17587820 Review.
-
TGF-beta signalling and immunity in prostate tumourigenesis.Expert Opin Ther Targets. 2010 Feb;14(2):179-92. doi: 10.1517/14728220903544507. Expert Opin Ther Targets. 2010. PMID: 20055717 Review.
Cited by
-
Transforming growth factor-β1 induces connective tissue growth factor expression and promotes peritoneal metastasis of gastric cancer.Biosci Rep. 2020 Sep 30;40(9):BSR20201501. doi: 10.1042/BSR20201501. Biosci Rep. 2020. PMID: 32885819 Free PMC article.
-
Altered expression pattern of immune response-related genes and isoforms in hypersensitivity pneumonitis lung fibroblasts.Sci Rep. 2024 Oct 14;14(1):24002. doi: 10.1038/s41598-024-74267-x. Sci Rep. 2024. PMID: 39402115 Free PMC article.
-
Transcriptome Bioinformatical Analysis of Vertebrate Stages of Schistosoma japonicum Reveals Alternative Splicing Events.PLoS One. 2015 Sep 25;10(9):e0138470. doi: 10.1371/journal.pone.0138470. eCollection 2015. PLoS One. 2015. PMID: 26407301 Free PMC article.
-
Regulation of the regenerative activity of dental pulp stem cells from exfoliated deciduous teeth (SHED) of children by TGF-β1 is associated with ALK5/Smad2, TAK1, p38 and MEK/ERK signaling.Aging (Albany NY). 2020 Nov 4;12(21):21253-21272. doi: 10.18632/aging.103848. Epub 2020 Nov 4. Aging (Albany NY). 2020. PMID: 33148869 Free PMC article.
-
Alternative splicing in EMT and TGF-β signaling during cancer progression.Semin Cancer Biol. 2024 Jun;101:1-11. doi: 10.1016/j.semcancer.2024.04.001. Epub 2024 Apr 15. Semin Cancer Biol. 2024. PMID: 38614376 Free PMC article. Review.
References
-
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata M, Doetschmann T. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. - DOI - PMC - PubMed
-
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. - DOI - PMC - PubMed
-
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-β1 knock out mice. Development. 1995;121:1845–1854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

