Alternative splicing of TGF-betas and their high-affinity receptors T beta RI, T beta RII and T beta RIII (betaglycan) reveal new variants in human prostatic cells
- PMID: 17845732
- PMCID: PMC2075524
- DOI: 10.1186/1471-2164-8-318
Alternative splicing of TGF-betas and their high-affinity receptors T beta RI, T beta RII and T beta RIII (betaglycan) reveal new variants in human prostatic cells
Abstract
Background: The transforming growth factors (TGF)-beta, TGF-beta1, TGF-beta2 and TGF-beta 3, and their receptors [T beta RI, T beta RII, T beta R III (betaglycan)] elicit pleiotropic functions in the prostate. Although expression of the ligands and receptors have been investigated, the splice variants have never been analyzed. We therefore have analyzed all ligands, the receptors and the splice variants T beta RIB, T beta RIIB and TGF-beta 2B in human prostatic cells.
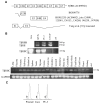
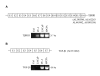
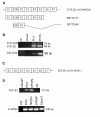
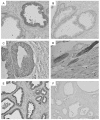
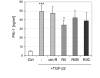
Results: Interestingly, a novel human receptor transcript T beta RIIC was identified, encoding additional 36 amino acids in the extracellular domain, that is expressed in the prostatic cancer cells PC-3, stromal hPCPs, and other human tissues. Furthermore, the receptor variant T beta RIB with four additional amino acids was identified also in human. Expression of the variant T beta RIIB was found in all prostate cell lines studied with a preferential localization in epithelial cells in some human prostatic glands. Similarly, we observed localization of T beta RIIC and TGF-beta 2B mainly in the epithelial cells with a preferential localization of TGF-beta 2B in the apical cell compartment. Whereas in the androgen-independent hPCPs and PC-3 cells all TGF-beta ligands and receptors are expressed, the androgen-dependent LNCaP cells failed to express all ligands. Additionally, stimulation of PC-3 cells with TGF-beta2 resulted in a significant and strong increase in secretion of plasminogen activator inhibitor-1 (PAI-1) with a major participation of T beta RII.
Conclusion: In general, expression of the splice variants was more heterogeneous in contrast to the well-known isoforms. The identification of the splice variants T beta RIB and the novel isoform T beta RIIC in man clearly contributes to the growing complexity of the TGF-beta family.
Figures


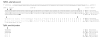

References
-
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata M, Doetschmann T. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. - DOI - PMC - PubMed
-
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. - DOI - PMC - PubMed
-
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-β1 knock out mice. Development. 1995;121:1845–1854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

