Presentation of Toxoplasma gondii antigens via the endogenous major histocompatibility complex class I pathway in nonprofessional and professional antigen-presenting cells
- PMID: 17846116
- PMCID: PMC2168266
- DOI: 10.1128/IAI.00954-07
Presentation of Toxoplasma gondii antigens via the endogenous major histocompatibility complex class I pathway in nonprofessional and professional antigen-presenting cells
Abstract
Challenge with the intracellular protozoan parasite Toxoplasma gondii induces a potent CD8+ T-cell response that is required for resistance to infection, but many questions remain about the factors that regulate the presentation of major histocompatibility complex class I (MHC-I)-restricted parasite antigens and about the role of professional and nonprofessional accessory cells. In order to address these issues, transgenic parasites expressing ovalbumin (OVA), reagents that track OVA/MHC-I presentation, and OVA-specific CD8+ T cells were exploited to compare the abilities of different infected cell types to stimulate CD8+ T cells and to define the factors that contribute to antigen processing. These studies reveal that a variety of infected cell types, including hematopoietic and nonhematopoietic cells, are capable of activating an OVA-specific CD8+ T-cell hybridoma, and that this phenomenon is dependent on the transporter associated with antigen processing and requires live T. gondii. Several experimental approaches indicate that T-cell activation is a consequence of direct presentation by infected host cells rather than cross-presentation. Surprisingly, nonprofessional antigen-presenting cells (APCs) were at least as efficient as dendritic cells at activating this MHC-I-restricted response. Studies to assess whether these cells are involved in initiation of the CD8+ T-cell response to T. gondii in vivo show that chimeric mice expressing MHC-I only in nonhematopoietic compartments are able to activate OVA-specific CD8+ T cells upon challenge. These findings associate nonprofessional APCs with the initial activation of CD8+ T cells during toxoplasmosis.
Figures

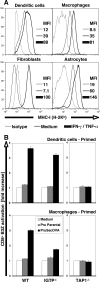
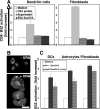

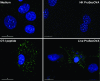
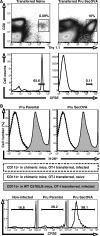
References
-
- Bertholet, S., R. Goldszmid, A. Morrot, A. Debrabant, F. Afrin, C. Collazo-Custodio, M. Houde, M. Desjardins, A. Sher, and D. Sacks. 2006. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J. Immunol. 177:3525-3533. - PubMed
-
- Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

