Clinical and pharmacological determinants of the therapeutic response to dihydroartemisinin-piperaquine for drug-resistant malaria
- PMID: 17846129
- PMCID: PMC2151469
- DOI: 10.1128/AAC.00486-07
Clinical and pharmacological determinants of the therapeutic response to dihydroartemisinin-piperaquine for drug-resistant malaria
Abstract
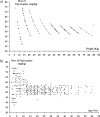
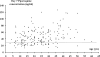
Dihydroartemisinin-piperaquine (DHP) is an important new treatment for drug-resistant malaria, although pharmacokinetic studies on the combination are limited. In Papua, Indonesia, we assessed determinants of the therapeutic efficacy of DHP for uncomplicated malaria. Plasma piperaquine concentrations were measured on day 7 and day 28, and the cumulative risk of parasitological failure at day 42 was calculated using survival analysis. Of the 598 patients in the evaluable population 342 had infections with Plasmodium falciparum, 83 with Plasmodium vivax, and 173 with a mixture of both species. The unadjusted cumulative risks of recurrence were 7.0% (95% confidence interval [CI]: 4.6 to 9.4%) for P. falciparum and 8.9% (95% CI: 6.0 to 12%) for P. vivax. After correcting for reinfections the risk of recrudescence with P. falciparum was 1.1% (95% CI: 0.1 to 2.1%). The major determinant of parasitological failure was the plasma piperaquine concentration. A concentration below 30 ng/ml on day 7 was observed in 38% (21/56) of children less than 15 years old and 22% (31/140) of adults (P = 0.04), even though the overall dose (mg per kg of body weight) in children was 9% higher than that in adults (P < 0.001). Patients with piperaquine levels below 30 ng/ml were more likely to have a recurrence with P. falciparum (hazard ratio [HR] = 6.6 [95% CI: 1.9 to 23]; P = 0.003) or P. vivax (HR = 9.0 [95% CI: 2.3 to 35]; P = 0.001). The plasma concentration of piperaquine on day 7 was the major determinant of the therapeutic response to DHP. Lower plasma piperaquine concentrations and higher failure rates in children suggest that dose revision may be warranted in this age group.
Figures

References
-
- Ashley, E. A., S. Krudsood, L. Phaiphun, S. Srivilairit, R. McGready, W. Leowattana, R. Hutagalung, P. Wilairatana, A. Brockman, S. Looareesuwan, F. Nosten, and N. J. White. 2004. Randomized, controlled dose-optimization studies of dihydroartemisinin-piperaquine for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. J. Infect. Dis. 190:1773-1782. - PubMed
-
- Barnes, K. I., F. Little, P. J. Smith, A. Evans, W. M. Watkins, and N. J. White. 2006. Sulfadoxine-pyrimethamine pharmacokinetics in malaria: pediatric dosing implications. Clin. Pharmacol. Ther. 80:582-596. - PubMed
-
- Brockman, A., R. E. Paul, T. J. Anderson, I. Hackford, L. Phaiphun, S. Looareesuwan, F. Nosten, and K. P. Day. 1999. Application of genetic markers to the identification of recrudescent Plasmodium falciparum infections on the northwestern border of Thailand. Am. J. Trop. Med. Hyg. 60:14-21. - PubMed
-
- Davis, T. M., T. Y. Hung, I. K. Sim, H. A. Karunajeewa, and K. F. Ilett. 2005. Piperaquine: a resurgent antimalarial drug. Drugs 65:75-87. - PubMed
-
- Denis, M. B., T. M. Davis, S. Hewitt, S. Incardona, K. Nimol, T. Fandeur, Y. Poravuth, C. Lim, and D. Socheat. 2002. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin. Infect. Dis. 35:1469-1476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

