Small-molecule scaffolds for CYP51 inhibitors identified by high-throughput screening and defined by X-ray crystallography
- PMID: 17846131
- PMCID: PMC2151439
- DOI: 10.1128/AAC.00311-07
Small-molecule scaffolds for CYP51 inhibitors identified by high-throughput screening and defined by X-ray crystallography
Abstract
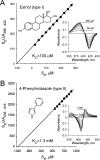
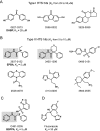
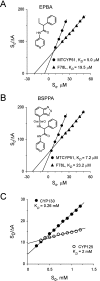
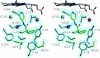
Sterol 14alpha-demethylase (CYP51), a major checkpoint in membrane sterol biosynthesis, is a key target for fungal antibiotic therapy. We sought small organic molecules for lead candidate CYP51 inhibitors. The changes in CYP51 spectral properties following ligand binding make CYP51 a convenient target for high-throughput screening technologies. These changes are characteristic of either substrate binding (type I) or inhibitor binding (type II) in the active site. We screened a library of 20,000 organic molecules against Mycobacterium tuberculosis CYP51 (CYP51(Mt)), examined the top type I and type II binding hits for their inhibitory effects on M. tuberculosis in broth culture, and analyzed them spectrally for their ability to discriminate between CYP51(Mt) and two reference M. tuberculosis CYP proteins, CYP130 and CYP125. We determined the binding mode for one of the top type II hits, alpha-ethyl-N-4-pyridinyl-benzeneacetamide (EPBA), by solving the X-ray structure of the CYP51(Mt)-EPBA complex to a resolution of 1.53 A. EPBA binds coordinately to the heme iron in the CYP51(Mt) active site through a lone pair of nitrogen electrons and also through hydrogen bonds with residues H259 and Y76, which are invariable in the CYP51 family, and hydrophobic interactions in a phylum- and/or substrate-specific cavity of CYP51. We also identified a second compound with structural and binding properties similar to those of EPBA, 2-(benzo[d]-2,1,3-thiadiazole-4-sulfonyl)-2-amino-2-phenyl-N-(pyridinyl-4)-acetamide (BSPPA). The congruence between the geometries of EPBA and BSPPA and the CYP51 binding site singles out EPBA and BSPPA as lead candidate CYP51 inhibitors with optimization potential for efficient discrimination between host and pathogen enzymes.
Figures



References
-
- Abe, I., Y. F. Zheng, and G. D. Prestwich. 1998. Photoaffinity labeling of oxidosqualene cyclase and squalene cyclase by a benzophenone-containing inhibitor. Biochemistry 37:5779-5784. - PubMed
-
- Aoyama, Y. 2005. Recent progress in the CYP51 research focusing on its unique evolutionary and functional characteristics as a diversozyme P450. Front. Biosci. 10:1546-1557. - PubMed
-
- Aoyama, Y., T. Horiuchi, O. Gotoh, M. Noshiro, and Y. Yoshida. 1998. CYP51-like gene of Mycobacterium tuberculosis actually encodes a P450 similar to eukaryotic CYP51. J. Biochem. (Tokyo) 124:694-696. - PubMed
-
- Brunger, A. T., P. D. Adams, G. M. Clore, W. L. Delano, P. Gros, R. W. Grosse-Kunstleve, J.-S. Jiang, J. Kuszewski, M. Nilges, and N. S. Pannu. 1998. Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54:905-921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Molecular Biology Databases

