Functionally specialized junctions between endothelial cells of lymphatic vessels
- PMID: 17846148
- PMCID: PMC2118470
- DOI: 10.1084/jem.20062596
Functionally specialized junctions between endothelial cells of lymphatic vessels
Abstract
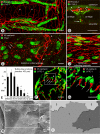
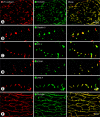
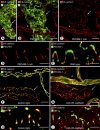
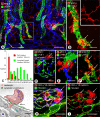
Recirculation of fluid and cells through lymphatic vessels plays a key role in normal tissue homeostasis, inflammatory diseases, and cancer. Despite recent advances in understanding lymphatic function (Alitalo, K., T. Tammela, and T.V. Petrova. 2005. Nature. 438:946-953), the cellular features responsible for entry of fluid and cells into lymphatics are incompletely understood. We report the presence of novel junctions between endothelial cells of initial lymphatics at likely sites of fluid entry. Overlapping flaps at borders of oak leaf-shaped endothelial cells of initial lymphatics lacked junctions at the tip but were anchored on the sides by discontinuous button-like junctions (buttons) that differed from conventional, continuous, zipper-like junctions (zippers) in collecting lymphatics and blood vessels. However, both buttons and zippers were composed of vascular endothelial cadherin (VE-cadherin) and tight junction-associated proteins, including occludin, claudin-5, zonula occludens-1, junctional adhesion molecule-A, and endothelial cell-selective adhesion molecule. In C57BL/6 mice, VE-cadherin was required for maintenance of junctional integrity, but platelet/endothelial cell adhesion molecule-1 was not. Growing tips of lymphatic sprouts had zippers, not buttons, suggesting that buttons are specialized junctions rather than immature ones. Our findings suggest that fluid enters throughout initial lymphatics via openings between buttons, which open and close without disrupting junctional integrity, but most leukocytes enter the proximal half of initial lymphatics.
Figures


References
-
- Witte, M.H., K. Jones, J. Wilting, M. Dictor, M. Selg, N. McHale, J.E. Gershenwald, and D.G. Jackson. 2006. Structure function relationships in the lymphatic system and implications for cancer biology. Cancer Metastasis Rev. 25:159–184. - PubMed
-
- Ji, R.C. 2006. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: New insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 25:677–694. - PubMed
-
- Tobler, N.E., and M. Detmar. 2006. Tumor and lymph node lymphangiogenesis–impact on cancer metastasis. J. Leukoc. Biol. 80:691–696. - PubMed
-
- Randolph, G.J., V. Angeli, and M.A. Swartz. 2005. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5:617–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

