The mechanism of fast-gate opening in ClC-0
- PMID: 17846164
- PMCID: PMC2151655
- DOI: 10.1085/jgp.200709759
The mechanism of fast-gate opening in ClC-0
Abstract
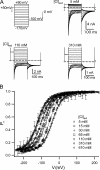
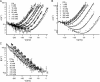
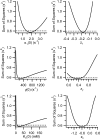
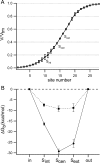
ClC-0 is a chloride channel whose gating is sensitive to both voltage and chloride. Based on analysis of gating kinetics using single-channel recordings, a five-state model was proposed to describe the dependence of ClC-0 fast-gate opening on voltage and external chloride (Chen, T.-Y., and C. Miller. 1996. J. Gen. Physiol. 108:237-250). We aimed to use this five-state model as a starting point for understanding the structural changes that occur during gating. Using macroscopic patch recordings, we were able to reproduce the effects of voltage and chloride that were reported by Chen and Miller and to fit our opening rate constant data to the five-state model. Upon further analysis of both our data and those of Chen and Miller, we learned that in contrast to their conclusions, (a) the features in the data are not adequate to rule out a simpler four-state model, and (b) the chloride-binding step is voltage dependent. In order to be able to evaluate the effects of mutants on gating (described in the companion paper, see Engh et al. on p. 351 of this issue), we developed a method for determining the error on gating model parameters, and evaluated the sources of this error. To begin to mesh the kinetic model(s) with the known CLC structures, a model of ClC-0 was generated computationally based on the X-ray crystal structure of the prokaryotic homolog ClC-ec1. Analysis of pore electrostatics in this homology model suggests that at least two of the conclusions derived from the gating kinetics analysis are consistent with the known CLC structures: (1) chloride binding is necessary for channel opening, and (2) chloride binding to any of the three known chloride-binding sites must be voltage dependent.
Figures


Similar articles
-
The role of a conserved lysine in chloride- and voltage-dependent ClC-0 fast gating.J Gen Physiol. 2007 Oct;130(4):351-63. doi: 10.1085/jgp.200709760. Epub 2007 Sep 10. J Gen Physiol. 2007. PMID: 17846165 Free PMC article.
-
Proton-dependent inhibition, inverted voltage activation, and slow gating of CLC-0 Chloride Channel.PLoS One. 2020 Dec 23;15(12):e0240704. doi: 10.1371/journal.pone.0240704. eCollection 2020. PLoS One. 2020. PMID: 33362212 Free PMC article.
-
Removal of gating in voltage-dependent ClC-2 chloride channel by point mutations affecting the pore and C-terminus CBS-2 domain.J Physiol. 2006 Apr 1;572(Pt 1):173-81. doi: 10.1113/jphysiol.2005.102392. Epub 2006 Feb 9. J Physiol. 2006. PMID: 16469788 Free PMC article.
-
Coupling gating with ion permeation in ClC channels.Sci STKE. 2003 Jun 24;2003(188):pe23. doi: 10.1126/stke.2003.188.pe23. Sci STKE. 2003. PMID: 12824475 Review.
-
Structural insights into chloride and proton-mediated gating of CLC chloride channels.Biochemistry. 2004 Feb 10;43(5):1135-44. doi: 10.1021/bi0359776. Biochemistry. 2004. PMID: 14756549 Review.
Cited by
-
Revealing the activation pathway for TMEM16A chloride channels from macroscopic currents and kinetic models.Pflugers Arch. 2016 Jul;468(7):1241-1257. doi: 10.1007/s00424-016-1830-9. Epub 2016 May 2. Pflugers Arch. 2016. PMID: 27138167 Free PMC article.
-
Basis of substrate binding and conservation of selectivity in the CLC family of channels and transporters.Nat Struct Mol Biol. 2009 Dec;16(12):1294-301. doi: 10.1038/nsmb.1704. Epub 2009 Nov 8. Nat Struct Mol Biol. 2009. PMID: 19898476 Free PMC article.
-
The role of a conserved lysine in chloride- and voltage-dependent ClC-0 fast gating.J Gen Physiol. 2007 Oct;130(4):351-63. doi: 10.1085/jgp.200709760. Epub 2007 Sep 10. J Gen Physiol. 2007. PMID: 17846165 Free PMC article.
-
On the mechanism of gating charge movement of ClC-5, a human Cl(-)/H(+) antiporter.Biophys J. 2012 May 2;102(9):2060-9. doi: 10.1016/j.bpj.2012.03.067. Biophys J. 2012. PMID: 22824269 Free PMC article.
-
Dynamical model of the CLC-2 ion channel reveals conformational changes associated with selectivity-filter gating.PLoS Comput Biol. 2020 Mar 30;16(3):e1007530. doi: 10.1371/journal.pcbi.1007530. eCollection 2020 Mar. PLoS Comput Biol. 2020. PMID: 32226009 Free PMC article.
References
-
- Accardi, A., S. Lobet, C. Williams, C. Miller, and R. Dutzler. 2006. Synergism between halide binding and proton transport in a CLC-type exhanger. J. Mol. Biol. 362:691–699. - PubMed
-
- Barnett, V., and T. Lewis. 1994. Outliers in Statistical Data. Third ed. John Wiley and Sons, New York. 164.
-
- Bell, S.P., P.K. Curran, S. Choi, and J.A. Mindell. 2006. Site-directed fluorescence studies of a prokaryotic ClC antiporter. Biochemistry. 45:6773–6782. - PubMed

