Energetics of water permeation through fullerene membrane
- PMID: 17846427
- PMCID: PMC1975687
- DOI: 10.1073/pnas.0705010104
Energetics of water permeation through fullerene membrane
Abstract
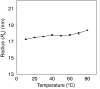
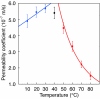
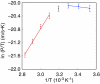
Lipid bilayer membranes are important as fundamental structures in biology and possess characteristic water-permeability, stability, and mechanical properties. Water permeation through a lipid bilayer membrane occurs readily, and more readily at higher temperature, which is largely due to an enthalpy cost of the liquid-to-gas phase transition of water. A fullerene bilayer membrane formed by dissolution of a water-soluble fullerene, Ph(5)C(60)K, has now been shown to possess properties entirely different from those of the lipid membranes. The fullerene membrane is several orders of magnitude less permeable to water than a lipid membrane, and the permeability decreases at higher temperature. Water permeation is burdened by a very large entropy loss and may be favored slightly by an enthalpy gain, which is contrary to the energetics observed for the lipid membrane. We ascribe this energetics to favorable interactions of water molecules to the surface of the fullerene molecules as they pass through the clefts of the rigid fullerene bilayer. The findings provide possibilities of membrane design in science and technology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Passive transport of C60 fullerenes through a lipid membrane: a molecular dynamics simulation study.J Phys Chem B. 2008 Feb 21;112(7):2078-84. doi: 10.1021/jp075149c. Epub 2008 Jan 30. J Phys Chem B. 2008. PMID: 18229908
-
Distribution of Fullerene Nanoparticles between Water and Solid Supported Lipid Membranes: Thermodynamics and Effects of Membrane Composition on Distribution.Environ Sci Technol. 2015 Dec 15;49(24):14546-53. doi: 10.1021/acs.est.5b03339. Epub 2015 Dec 2. Environ Sci Technol. 2015. PMID: 26569041
-
Distribution of fullerene nanomaterials between water and model biological membranes.Langmuir. 2011 Oct 4;27(19):11899-905. doi: 10.1021/la2017837. Epub 2011 Sep 9. Langmuir. 2011. PMID: 21854052
-
Photodynamic Activity of Fullerenes and Other Molecules Incorporated into Lipid Membranes by Exchange.Chem Rec. 2016 Feb;16(1):249-60. doi: 10.1002/tcr.201500249. Epub 2015 Dec 12. Chem Rec. 2016. PMID: 26663769 Review.
-
Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience.Acc Chem Res. 2003 Nov;36(11):807-15. doi: 10.1021/ar030027y. Acc Chem Res. 2003. PMID: 14622027 Review.
Cited by
-
The Effect of Different Substances Embedded in Fullerene Cavity on Surfactant Self-Assembly Behavior through Molecular Dynamics Simulation.Molecules. 2024 May 16;29(10):2355. doi: 10.3390/molecules29102355. Molecules. 2024. PMID: 38792216 Free PMC article.
-
Phenine design for nanocarbon molecules.Proc Jpn Acad Ser B Phys Biol Sci. 2022;98(8):379-400. doi: 10.2183/pjab.98.020. Proc Jpn Acad Ser B Phys Biol Sci. 2022. PMID: 36216532 Free PMC article.
References
-
- Gennis RB. Biomembranes: Molecular Structure and Function. New York: Springer; 1989.
-
- Haines TH, Liebovitch LS. In: Permeability and Stability of Lipid Bilayers. Disalvo EA, Simon SA, editors. Boca Raton, FL: CRC; 1995. pp. 123–136.
-
- Matsuo Y, Tahara K, Nakamura E. Chem Lett. 2005;34:1078–1079.
-
- Sawamura M, Nagahama N, Toganoh M, Hackler UE, Isobe H, Nakamura E, Zhou SQ, Chu B. Chem Lett. 2000;29:1098–1099. - PubMed
-
- Zhou SQ, Burger C, Chu B, Sawamura M, Nagahama N, Toganoh M, Hackler UE, Isobe H, Nakamura E. Science. 2001;291:1944–1947. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

