Evaluation of phenoxymethylpenicillin treatment of acute otitis media in children aged 2-16
- PMID: 17846935
- PMCID: PMC3379776
- DOI: 10.1080/02813430701267405
Evaluation of phenoxymethylpenicillin treatment of acute otitis media in children aged 2-16
Abstract
Objective: To study the clinical recovery from acute otitis media (AOM) in children, 2-16 years of age, managed with or without treatment with phenoxymethylpenicillin (PcV).
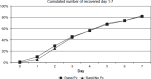
Design: An open, prospective randomized trial. Children aged between 2 and 16 years, presenting with one- or double-sided AOM (without perforation) with symptom duration of less than four days, were included. The children were randomized to PcV for five days or to no primary antibiotic treatment. A health score and compliance were registered on a daily basis for seven days.
Setting: A total of 32 health centres and 72 GPs in south-east Sweden. Subjects. Children aged 2-16 presenting with earache.
Main outcome measures: Recovery time, symptom duration, frequency of complications (up to three months) and consumption of healthcare services independent of treatment with or without antibiotics.
Results: A total of 179 patients carried out the trial; 92 were randomized to PcV, 87 to no primary antibiotic treatment. The median recovery time was four days in both groups. Patients who received PcV had less pain (p <0.001) and used fewer analgesics. There were no significant differences in the number of middle-ear effusions or perforations at the final control after three months. Children randomized to PcV treatment consulted less (p <0.001) during the first seven days.
Conclusions: Our investigation supports that PcV treatment of AOM does not affect the recovery time or complication rates. PcV provided some symptomatic benefit in the treatment of AOM in otherwise healthy children, aged 2-16 years.
Figures
References
-
- Mygind N, Meistrup-Larsen KI, Thomsen J, Thomsen VF, Josefsson K, Sorensen H. Penicillin in acute otitis media: A double-blind placebo-controlled trial. Clinical Otolaryngology & Allied Sciences. 1981;6:5–13. - PubMed
-
- Rosenfeld RM, Vertrees JE, Carr J, Cipolle RJ, Uden DL, Giebink GS, et al. Clinical efficacy of antimicrobial drugs for acute otitis media: Metaanalysis of 5400 children from thirty-three randomized trials [see comment] J Pediatr. 1994;124:355–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical