Clinical and molecular phenotype of Aicardi-Goutieres syndrome
- PMID: 17846997
- PMCID: PMC2227922
- DOI: 10.1086/521373
Clinical and molecular phenotype of Aicardi-Goutieres syndrome
Abstract
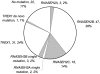
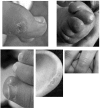
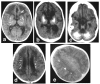
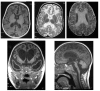
Aicardi-Goutieres syndrome (AGS) is a genetic encephalopathy whose clinical features mimic those of acquired in utero viral infection. AGS exhibits locus heterogeneity, with mutations identified in genes encoding the 3'-->5' exonuclease TREX1 and the three subunits of the RNASEH2 endonuclease complex. To define the molecular spectrum of AGS, we performed mutation screening in patients, from 127 pedigrees, with a clinical diagnosis of the disease. Biallelic mutations in TREX1, RNASEH2A, RNASEH2B, and RNASEH2C were observed in 31, 3, 47, and 18 families, respectively. In five families, we identified an RNASEH2A or RNASEH2B mutation on one allele only. In one child, the disease occurred because of a de novo heterozygous TREX1 mutation. In 22 families, no mutations were found. Null mutations were common in TREX1, although a specific missense mutation was observed frequently in patients from northern Europe. Almost all mutations in RNASEH2A, RNASEH2B, and RNASEH2C were missense. We identified an RNASEH2C founder mutation in 13 Pakistani families. We also collected clinical data from 123 mutation-positive patients. Two clinical presentations could be delineated: an early-onset neonatal form, highly reminiscent of congenital infection seen particularly with TREX1 mutations, and a later-onset presentation, sometimes occurring after several months of normal development and occasionally associated with remarkably preserved neurological function, most frequently due to RNASEH2B mutations. Mortality was correlated with genotype; 34.3% of patients with TREX1, RNASEH2A, and RNASEH2C mutations versus 8.0% RNASEH2B mutation-positive patients were known to have died (P=.001). Our analysis defines the phenotypic spectrum of AGS and suggests a coherent mutation-screening strategy in this heterogeneous disorder. Additionally, our data indicate that at least one further AGS-causing gene remains to be identified.
Figures


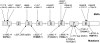
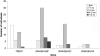
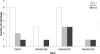
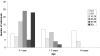
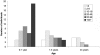
References
Web Resources
-
- GenBank, http://www.ncbi.nlm.nih.gov/GenBank/ (for TREX1 protein [transcript AAK07616 and nucleotide sequence NM_033627, with the A at 2986 as the first base of the initiating ATG codon], RNASEH2A protein [transcript AAH11748.1 and nucleotide sequence NM_006397.2], RNASEH2B protein [transcript AAH36744.1 and nucleotide sequence NM_024570.1], and RNASEH2C protein [accession number AAH23588.1 and nucleotide sequence NM_032193.3])
-
- International Aicardi-Goutières Syndrome Association, http://www.aicardi-goutieres.org/
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CSF lymphocytosis, TREX1, RNASEH2A, RNASEH2B, RNASEH2C, Alexander disease, vanishing white-matter disease, SLE, and familial chilblain lupus)
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

