Activation of G protein-coupled receptors: beyond two-state models and tertiary conformational changes
- PMID: 17848137
- PMCID: PMC2639654
- DOI: 10.1146/annurev.pharmtox.48.113006.094630
Activation of G protein-coupled receptors: beyond two-state models and tertiary conformational changes
Abstract
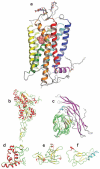
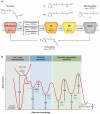
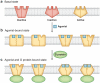
Transformation of G protein-coupled receptors (GPCRs) from a quiescent to an active state initiates signal transduction. All GPCRs share a common architecture comprising seven transmembrane-spanning alpha-helices, which accommodates signal propagation from a diverse repertoire of external stimuli across biological membranes to a heterotrimeric G protein. Signal propagation through the transmembrane helices likely involves mechanistic features common to all GPCRs. The structure of the light receptor rhodopsin may serve as a prototype for the transmembrane architecture of GPCRs. Early biochemical, biophysical, and pharmacological studies led to the conceptualization of receptor activation based on the context of two-state equilibrium models and conformational changes in protein structure. More recent studies indicate a need to move beyond these classical paradigms and to consider additional aspects of the molecular character of GPCRs, such as the oligomerization and dynamics of the receptor.
Figures
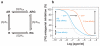


References
LITERATURE CITED
-
- Fredriksson R, Schioth HB. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol. Pharmacol. 2005;67:1414–25. - PubMed
-
- Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol. Rev. 2005;57:279–88. - PubMed
-
- Ballesteros JA, Shi L, Javitch JA. Structural mimicry in G protein-coupled receptors: implications of the high-resolution structure of rhodopsin for structure-function analysis of rhodopsin-like receptors. Mol. Pharmacol. 2001;60:1–19. - PubMed
RELATED RESOURCES
-
- Kenakin T. Drug efficacy at G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2002;42:349–79. - PubMed
-
- May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein–coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2007;47:1–51. - PubMed
-
- Smit MJ, Vischer HF, Bakker RA, Jongejan A, Timmerman H, et al. Pharmacogenomic and structural analysis of constitutive G protein–coupled receptor activity. Annu. Rev. Pharmacol. Toxicol. 2007;47:53–87. - PubMed
-
- Spiegel AM, Weinstein LS. Inherited diseases involving G proteins and G protein–coupled receptors. Annu. Rev. Med. 2004;55:27–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

