Gap junction protein expression and cellularity: comparison of immature and adult equine digital tendons
- PMID: 17848160
- PMCID: PMC2375813
- DOI: 10.1111/j.1469-7580.2007.00781.x
Gap junction protein expression and cellularity: comparison of immature and adult equine digital tendons
Abstract
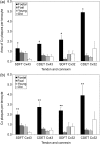
Injury to the energy-storing superficial digital flexor tendon is common in equine athletes and is age-related. Tenocytes in the superficial digital flexor tendon of adult horses appear to have limited ability to respond adaptively to exercise or prevent the accumulation of strain-induced microdamage. It has been suggested that conditioning exercise should be introduced during the growth period, when tenocytes may be more responsive to increased quantities or intensities of mechanical strain. Tenocytes are linked into networks by gap junctions that allow coordination of synthetic activity and facilitate strain-induced collagen synthesis. We hypothesised that there are reductions in cellular expression of the gap junction proteins connexin (Cx) 43 and 32 during maturation and ageing of the superficial digital flexor tendon that do not occur in the non-injury-prone common digital extensor tendon. Cryosections from the superficial digital flexor tendon and common digital extensor tendon of 5 fetuses, 5 foals (1-6 months), 5 young adults (2-7 years) and 5 old horses (18-33 years) were immunofluorescently labelled and quantitative confocal laser microscopy was performed. Expression of Cx43 and Cx32 protein per tenocyte was significantly higher in the fetal group compared with all other age groups in both tendons. The density of tenocytes was found to be highest in immature tissue. Higher levels of cellularity and connexin protein expression in immature tendons are likely to relate to requirements for tissue remodelling and growth. However, if further studies demonstrate that this correlates with greater gap junctional communication efficiency and synthetic responsiveness to mechanical strain in immature compared with adult tendons, it could support the concept of early introduction of controlled exercise as a means of increasing resistance to later injury.
Figures





References
-
- Andrade-Rozental AF, Rozental R, Hopperstad MG, Wu JK, Vrionis FD, Spray DC. Gap junctions: the ‘kiss of death’ and the ‘kiss of life’. Brain Res Rev. 2000;32:308–315. - PubMed
-
- Arita K, Akiyama M, Tsuji Y, et al. Changes in gap junction distribution and connexin expression pattern during human fetal skin development. J Histochem Cytochem. 2002;50:1493–500. - PubMed
-
- Banes AJ, Tsuzaki M, Yamamoto J, et al. Trans Orthop Res Soc. Vol. 21. Atlanta: 1996. Connexin expression is upregulated by mechanical load in avian and human tendon cells; pp. 3–1.
-
- Banes AJ, Weinhold P, Yang X, et al. Gap junctions regulate responses of tendon cells ex vivo to mechanical loading. Clin Orthop. 1999:356–370. - PubMed
-
- Batson EL, Paramour RJ, Smith TJ, et al. Are the material properties and matrix composition of equine flexor and extensor tendons determined by their functions? Equine Vet J. 2003;35:314–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

