Structural basis for evasion of IgA immunity by Staphylococcus aureus revealed in the complex of SSL7 with Fc of human IgA1
- PMID: 17848512
- PMCID: PMC1986611
- DOI: 10.1073/pnas.0706028104
Structural basis for evasion of IgA immunity by Staphylococcus aureus revealed in the complex of SSL7 with Fc of human IgA1
Abstract
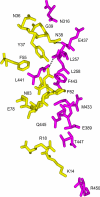
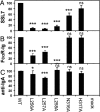
Infection by Staphylococcus aureus can result in severe conditions such as septicemia, toxic shock, pneumonia, and endocarditis with antibiotic resistance and persistent nasal carriage in normal individuals being key drivers of the medical impact of this virulent pathogen. In both virulent infection and nasal colonization, S. aureus encounters the host immune system and produces a wide array of factors that frustrate host immunity. One in particular, the prototypical staphylococcal superantigen-like protein SSL7, potently binds IgA and C5, thereby inhibiting immune responses dependent on these major immune mediators. We report here the three-dimensional structure of the complex of SSL7 with Fc of human IgA1 at 3.2 A resolution. Two SSL7 molecules interact with the Fc (one per heavy chain) primarily at the junction between the Calpha2 and Calpha3 domains. The binding site on each IgA chain is extensive, with SSL7 shielding most of the lateral surface of the Calpha3 domain. However, the SSL7 molecules are positioned such that they should allow binding to secretory IgA. The key IgA residues interacting with SSL7 are also bound by the leukocyte IgA receptor, FcalphaRI (CD89), thereby explaining how SSL7 potently inhibits IgA-dependent cellular effector functions mediated by FcalphaRI, such as phagocytosis, degranulation, and respiratory burst. Thus, the ability of S. aureus to subvert IgA-mediated immunity is likely to facilitate survival in mucosal environments such as the nasal passage and may contribute to systemic infections.
Conflict of interest statement
Conflict of interest statement: SSL7 is the subject of a filed provisional patent application.
Figures
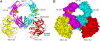


Similar articles
-
A competitive mechanism for staphylococcal toxin SSL7 inhibiting the leukocyte IgA receptor, Fc alphaRI, is revealed by SSL7 binding at the C alpha2/C alpha3 interface of IgA.J Biol Chem. 2006 Jan 20;281(3):1389-93. doi: 10.1074/jbc.M509334200. Epub 2005 Nov 17. J Biol Chem. 2006. PMID: 16293625
-
The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-Fc alpha RI binding and serum killing of bacteria.J Immunol. 2005 Mar 1;174(5):2926-33. doi: 10.4049/jimmunol.174.5.2926. J Immunol. 2005. PMID: 15728504
-
Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc.Nature. 2003 Jun 5;423(6940):614-20. doi: 10.1038/nature01685. Epub 2003 May 21. Nature. 2003. PMID: 12768205
-
IgA and the IgA Fc receptor.Trends Immunol. 2001 Apr;22(4):205-11. doi: 10.1016/s1471-4906(01)01873-7. Trends Immunol. 2001. PMID: 11274926 Review.
-
The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease.Immunol Rev. 2015 Nov;268(1):123-38. doi: 10.1111/imr.12337. Immunol Rev. 2015. PMID: 26497517 Review.
Cited by
-
Are anti-HIV IgAs good guys or bad guys?Retrovirology. 2014 Dec 14;11:109. doi: 10.1186/s12977-014-0109-5. Retrovirology. 2014. PMID: 25499540 Free PMC article. Review.
-
Enterococcus faecalis Promotes Innate Immune Suppression and Polymicrobial Catheter-Associated Urinary Tract Infection.Infect Immun. 2017 Nov 17;85(12):e00378-17. doi: 10.1128/IAI.00378-17. Print 2017 Dec. Infect Immun. 2017. PMID: 28893918 Free PMC article.
-
Exploring Immunome and Microbiome Interplay in Reproductive Health: Current Knowledge, Challenges, and Novel Diagnostic Tools.Semin Reprod Med. 2023 Sep;41(5):172-189. doi: 10.1055/s-0043-1778017. Epub 2024 Jan 23. Semin Reprod Med. 2023. PMID: 38262441 Free PMC article. Review.
-
Antigen binding to secretory immunoglobulin A results in decreased sensitivity to intestinal proteases and increased binding to cellular Fc receptors.J Biol Chem. 2010 Jan 8;285(2):953-60. doi: 10.1074/jbc.M109.059220. Epub 2009 Nov 12. J Biol Chem. 2010. PMID: 19910466 Free PMC article.
-
Substrate recognition by complement convertases revealed in the C5-cobra venom factor complex.EMBO J. 2011 Feb 2;30(3):606-16. doi: 10.1038/emboj.2010.341. Epub 2011 Jan 7. EMBO J. 2011. PMID: 21217642 Free PMC article.
References
-
- Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Lancet. 2006;368:874–885. - PubMed
-
- Bishop EJ, Howden BP. Expert Opin Emerg Drugs. 2007;12:1–22. - PubMed
-
- Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Hook M, Etienne J, et al. Science. 2007;315:1130–1133. - PubMed
-
- Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJ, van Kessel KP, van Strijp JA. Nat Immunol. 2005;6:920–927. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

