Proteasomal selection of multiprotein complexes recruited by LIM homeodomain transcription factors
- PMID: 17848518
- PMCID: PMC1986602
- DOI: 10.1073/pnas.0703738104
Proteasomal selection of multiprotein complexes recruited by LIM homeodomain transcription factors
Erratum in
- Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):17239
Abstract
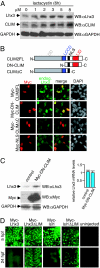
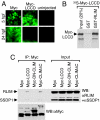
Complexes composed of multiple proteins regulate most cellular functions. However, our knowledge about the molecular mechanisms governing the assembly and dynamics of these complexes in cells remains limited. The in vivo activity of LIM homeodomain (LIM-HD) proteins, a class of transcription factors that regulates neuronal development, depends on the high-affinity association of their LIM domains with cofactor of LIM homeodomain proteins (LIM-HDs) (CLIM, also known as Ldb or NLI). CLIM cofactors recruit single-stranded DNA-binding protein 1 (SSDP1, also known as SSBP3), and this interaction is important for the activation of the LIM-HD/CLIM protein complex in vivo. Here, we identify a cascade of specific protein interactions that protect LIM-HD multiprotein complexes from proteasomal degradation. In this cascade, CLIM stabilizes LIM-HDs, and SSDP1 stabilizes CLIM. Furthermore, we show that stabilizing cofactors prevent binding of ubiquitin ligases to multiple protein interaction domains in LIM-HD recruited protein complexes. Together, our results indicate a combinatorial code that selects specific multiprotein complexes via proteasomal degradation in cells with broad implications for the assembly and specificity of multiprotein complexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Ubiquitination-dependent cofactor exchange on LIM homeodomain transcription factors.Nature. 2002 Mar 7;416(6876):99-103. doi: 10.1038/416099a. Nature. 2002. PMID: 11882901
-
SSDP cofactors regulate neural patterning and differentiation of specific axonal projections.Dev Biol. 2011 Jan 15;349(2):213-24. doi: 10.1016/j.ydbio.2010.10.037. Epub 2010 Nov 5. Dev Biol. 2011. PMID: 21056553 Free PMC article.
-
Multiple functions of LIM domain-binding CLIM/NLI/Ldb cofactors during zebrafish development.Mech Dev. 2002 Sep;117(1-2):75-85. doi: 10.1016/s0925-4773(02)00178-8. Mech Dev. 2002. PMID: 12204249
-
A short history of LIM domains (1993-2002): from protein interaction to degradation.Mol Neurobiol. 2002 Oct-Dec;26(2-3):269-81. doi: 10.1385/MN:26:2-3:269. Mol Neurobiol. 2002. PMID: 12428760 Review.
-
Competition between LIM-binding domains.Biochem Soc Trans. 2008 Dec;36(Pt 6):1393-7. doi: 10.1042/BST0361393. Biochem Soc Trans. 2008. PMID: 19021562 Review.
Cited by
-
Chondrolectin mediates growth cone interactions of motor axons with an intermediate target.J Neurosci. 2012 Mar 28;32(13):4426-39. doi: 10.1523/JNEUROSCI.5179-11.2012. J Neurosci. 2012. PMID: 22457492 Free PMC article.
-
LIM-domain transcription complexes interact with ring-finger ubiquitin ligases and thereby impact islet β-cell function.J Biol Chem. 2019 Aug 2;294(31):11728-11740. doi: 10.1074/jbc.RA118.006985. Epub 2019 Jun 11. J Biol Chem. 2019. PMID: 31186351 Free PMC article.
-
Ubiquitin and transcription: The SCF/Met4 pathway, a (protein-) complex issue.Transcription. 2011 May;2(3):135-139. doi: 10.4161/trns.2.3.15903. Transcription. 2011. PMID: 21826284 Free PMC article.
-
The Isl1/Ldb1 Complex Orchestrates Genome-wide Chromatin Organization to Instruct Differentiation of Multipotent Cardiac Progenitors.Cell Stem Cell. 2015 Sep 3;17(3):287-99. doi: 10.1016/j.stem.2015.08.007. Epub 2015 Aug 27. Cell Stem Cell. 2015. PMID: 26321200 Free PMC article.
-
The stage-dependent roles of Ldb1 and functional redundancy with Ldb2 in mammalian retinogenesis.Development. 2016 Nov 15;143(22):4182-4192. doi: 10.1242/dev.129734. Epub 2016 Oct 3. Development. 2016. PMID: 27697904 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

