Determinants of PF4/heparin immunogenicity
- PMID: 17848616
- PMCID: PMC2234783
- DOI: 10.1182/blood-2007-08-105098
Determinants of PF4/heparin immunogenicity
Abstract
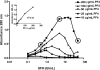
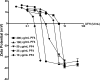
Heparin-induced thrombocytopenia (HIT) is an antibody-mediated disorder that occurs with variable frequency in patients exposed to heparin. HIT antibodies preferentially recognize large macromolecular complexes formed between PF4 and heparin over a narrow range of molar ratios, but the biophysical properties of complexes that initiate antibody production are unknown. To identify structural determinants underlying PF4/heparin immunogenicity, we characterized the in vitro interactions of murine PF4 (mPF4) and heparin with respect to light absorption, size, and surface charge (zeta potential). We show that PF4/heparin macromolecular assembly occurs through colloidal interactions, wherein heparin facilitates the growth of complexes through charge neutralization. The size of PF4/heparin macromolecules is governed by the molar ratios of the reactants. Maximal complex size occurs at molar ratios of PF4/heparin at which surface charge is neutral. When mice are immunized with complexes that differ in size and/or zeta potential, antibody formation varies inversely with heparin concentration and is most robust in animals immunized with complexes displaying a net positive zeta-potential. These studies suggest that the clinical heterogeneity in the HIT immune response may be due in part to requirements for specific biophysical parameters of the PF4/heparin complexes that occur in settings of intense platelet activation and PF4 release.
Figures
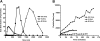

References
-
- Arepally GM, Ortel TL. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809–817. - PubMed
-
- Pouplard C, Couvret C, Regina S, Gruel Y. Development of antibodies specific to polyanion-modified platelet factor 4 during treatment with fondaparinux. J Thromb Haemost. 2005;3:2813–2815. - PubMed
-
- Visentin GP, Malik M, Cyganiak KA, Aster RH. Patients treated with unfractionated heparin during open heart surgery are at high risk to form antibodies reactive with heparin:platelet factor 4 complexes. J Lab Clin Med. 1996;128:376–383. - PubMed
-
- Bauer TL, Arepally G, Konkle BA, et al. Prevalence of heparin-associated antibodies without thrombosis in patients undergoing cardiopulmonary bypass surgery. Circulation. 1997;95:1242–1246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

