Antihuman factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation
- PMID: 17848617
- PMCID: PMC2234776
- DOI: 10.1182/blood-2007-06-096842
Antihuman factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation
Abstract
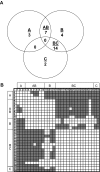
The diversity of factor VIII (fVIII) C2 domain antibody epitopes was investigated by competition enzyme-linked immunosorbent assay (ELISA) using a panel of 56 antibodies. The overlap patterns produced 5 groups of monoclonal antibodies (MAbs), designated A, AB, B, BC, and C, and yielded a set of 18 distinct epitopes. Group-specific loss of antigenicity was associated with mutations at the Met2199/Phe2200 phospholipid binding beta-hairpin (group AB MAbs) and at Lys2227 (group BC MAbs), which allowed orientation of the epitope structure as a continuum that covers one face of the C2 beta-sandwich. MAbs from groups A, AB, and B inhibit the binding of fVIIIa to phospholipid membranes. Group BC was the most common group and displayed the highest specific fVIII inhibitor activities. MAbs in this group are type II inhibitors that inhibit the activation of fVIII by either thrombin or factor Xa and poorly inhibit the binding of fVIII to phospholipid membranes or von Willebrand factor (VWF). Group BC MAbs are epitopically and mechanistically distinct from the extensively studied group C MAb, ESH8. These results reveal the structural and functional complexity of the anti-C2 domain antibody response and indicate that interference with fVIII activation is a major attribute of the inhibitor landscape.
Figures






References
-
- Lusher JM, Arkin S, Abildgaard CF, Schwartz RS the Kogenate Previously Untreated Patient Study Group. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A: safety, efficacy, and the development of inhibitors. N Engl J Med. 1993;328:453–459. - PubMed
-
- Bray GL, Gomperts ED, Courtier S, et al. A multicenter study of recombinant factor VIII (Recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. Blood. 1994;83:2428–2435. - PubMed
-
- Lusher JM, Lee CA, Kessler CM, Bedrosian CL the Refacto Phase 3 Study Group. The safety and efficacy of B-domain deleted recombinant factor VIII concentrate in patients with severe haemophilia A. Haemophilia. 2003;9:38–49. - PubMed
-
- Kreuz W, Ettingshausen CE, Zyschka A, et al. Inhibitor development in previously untreated patients with hemophilia A: a prospective long-term follow-up comparing plasma-derived and recombinant products. Semin Thromb Hemost. 2002;28:285–290. - PubMed
-
- Lollar P, Parker CG. Subunit structure of thrombin-activated porcine factor VIII. Biochemistry. 1989;28:666–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

