Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease
- PMID: 17848621
- PMCID: PMC2200820
- DOI: 10.1182/blood-2007-04-081703
Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease
Abstract
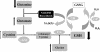
Erythrocyte glutathione depletion has been linked to hemolysis and oxidative stress. Glutamine plays an additional antioxidant role through preservation of intracellular nicotinamide adenine dinucleotide phosphate (NADPH) levels, required for glutathione recycling. Decreased nitric oxide (NO) bioavailability, which occurs in the setting of increased hemolysis and oxidative stress, contributes to the pathogenesis of pulmonary hypertension (PH) in sickle cell disease (SCD). We hypothesized that altered glutathione and glutamine metabolism play a role in this process. Total glutathione (and its precursors) and glutamine were assayed in plasma and erythrocytes of 40 SCD patients and 9 healthy volunteers. Erythrocyte total glutathione and glutamine levels were significantly lower in SCD patients than in healthy volunteers. Glutamine depletion was independently associated with PH, defined as a tricuspid regurgitant jet velocity (TRV) of at least 2.5 m/s. The ratio of erythrocyte glutamine:glutamate correlated inversely to TRV (r = -0.62, P < .001), plasma arginase concentration (r = -0.45, P = .002), and plasma-free hemoglobin level (r = -0.41, P = .01), linking erythrocyte glutamine depletion to dysregulation of the arginine-NO pathway and increased hemolytic rate. Decreased erythrocyte glutathione and glutamine levels contribute to alterations in the erythrocyte redox environment, which may compromise erythrocyte integrity, contribute to hemolysis, and play a role in the pathogenesis of PH of SCD.
Figures
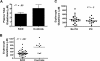

References
-
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–935. - PubMed
-
- Griffith OW. Glutathione turnover in human erythrocytes: inhibition by buthionine sulfoximine and incorporation of glycine by exchange. J Biol Chem. 1981;256:4900–4904. - PubMed
-
- Lunn G, Dale GL, Beutler E. Transport accounts for glutathione turnover in human erythrocytes. Blood. 1979;54:238–244. - PubMed
-
- Reid M, Badaloo A, Forrester T, et al. In vivo rates of erythrocyte glutathione synthesis in children with severe protein-energy malnutrition. Am J Physiol Endocrinol Metab. 2000;278:E405–E412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

