PPARgamma regulates adipose triglyceride lipase in adipocytes in vitro and in vivo
- PMID: 17848638
- PMCID: PMC2819189
- DOI: 10.1152/ajpendo.00122.2007
PPARgamma regulates adipose triglyceride lipase in adipocytes in vitro and in vivo
Abstract
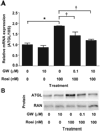
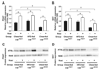
Peroxisome proliferator-activated receptor-gamma (PPARgamma) regulates adipocyte genes involved in adipogenesis and lipid metabolism and is the molecular target for thiazolidinedione (TZD) antidiabetic agents. Adipose triglyceride lipase (ATGL) is a recently described triglyceride-specific lipase that is induced during adipogenesis and remains highly expressed in mature adipocytes. This study evaluates the ability of PPARgamma to directly regulate ATGL expression in adipocytes in vitro and in vivo. In fully differentiated 3T3-L1 adipocytes, ATGL mRNA and protein are increased by TZD and non-TZD PPARgamma agonists in a dose- and time-dependent manner. Rosiglitazone-mediated induction of ATGL mRNA is rapid and is not inhibited by the protein synthesis inhibitor cycloheximide, indicating that intervening protein synthesis is not required for this effect. Rosiglitazone-mediated induction of ATGL mRNA and protein is inhibited by the PPARgamma-specific antagonist GW-9662 and is also significantly reduced following siRNA-mediated knockdown of PPARgamma, supporting the direct transcriptional regulation of ATGL by PPARgamma. In vivo, ATGL mRNA and protein are increased by rosiglitazone treatment in white and brown adipose tissue of mice with and without obesity due to high-fat diet or leptin deficiency. Thus, PPARgamma positively regulates ATGL mRNA and protein expression in mature adipocytes in vitro and in adipose tissue in vivo, suggesting a role for ATGL in mediating PPARgamma's effects on lipid metabolism.
Figures




References
-
- Arimura N, Horiba T, Imagawa M, Shimizu M, Sato R. The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J Biol Chem. 2004;279:10070–10076. - PubMed
-
- Baulande S, Lasnier F, Lucas M, Pairault J. Adiponutrin, a transmembrane protein corresponding to a novel dietary- and obesity-linked mRNA specifically expressed in the adipose lineage. J Biol Chem. 2001;276:33336–33344. - PubMed
-
- Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–478. - PubMed
-
- Bogacka I, Ukropcova B, McNeil M, Gimble JM, Smith SR. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. J Clin Endocrinol Metab. 2005;90:6650–6656. - PubMed
-
- Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1392–1399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

