Hypoxia-induced signaling in the cardiovascular system
- PMID: 17850210
- PMCID: PMC2871679
- DOI: 10.1146/annurev.physiol.70.113006.100526
Hypoxia-induced signaling in the cardiovascular system
Abstract
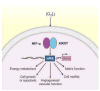
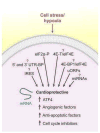
Low oxygen (O2) levels are a naturally occurring feature of embryonic development, adult physiology, and diseases such as those of the cardiovascular system. Although many responses to O2 deprivation are mediated by hypoxia-inducible factors (HIFs), researchers are finding a growing number of HIF-independent pathways that promote O2 conformance and hypoxia tolerance. Here, we describe HIF-independent responses and how they impact cardiovascular tissue homeostasis.
Figures


References
-
- Ramirez-Bergeron DL, Simon MC. Hypoxia-inducible factor and the development of stem cells of the cardiovascular system. Stem Cells. 2001;19:279–86. - PubMed
-
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–78. - PubMed
-
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54. - PubMed
-
- Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

