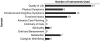A systematic review of measures of end-of-life care and its outcomes
- PMID: 17850523
- PMCID: PMC2254566
- DOI: 10.1111/j.1475-6773.2007.00721.x
A systematic review of measures of end-of-life care and its outcomes
Abstract
Objective: To identify psychometrically sound measures of outcomes in end-of-life care and to characterize their use in intervention studies.
Data sources: English language articles from 1990 to November 2005 describing measures with published psychometric data and intervention studies of end-of-life care.
Study design: Systematic review of end-of-life care literature.
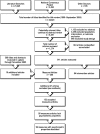
Extraction methods: Two reviewers organized identified measures into 10 major domains. Eight reviewers extracted and characterized measures from intervention studies.
Principal findings: Of 24,423 citations, we extracted 200 articles that described 261 measures, accepting 99 measures. In addition to 35 measures recommended in a prior systematic review, we identified an additional 64 measures of the end-of-life experience. The most robust measures were in the areas of symptoms, quality of life, and satisfaction; significant gaps existed in continuity of care, advance care planning, spirituality, and caregiver well-being. We also reviewed 84 intervention studies in which 135 patient-centered outcomes were assessed by 97 separate measures. Of these, 80 were used only once and only eight measures were used in more than two studies.
Conclusions: In general, most measures have not undergone rigorous development and testing. Measure development in end-of-life care should focus on areas with identified gaps, and testing should be done to facilitate comparability across the care settings, populations, and clinical conditions. Intervention research should use robust measures that adhere to these standards.
Figures
References
-
- Abratt R, Viljoen G. Assessment of Quality of Life by Clinicians—Experience of a Practical Method in Lung Cancer Patients. South Africa Medical Journal. 1995;85(9):896–8. - PubMed
-
- Bausewein C, Fegg M, Radbruch L, Nauck F, von Mackensen S, Borasio GD, Higginson IJ. Validation and Clinical Application of the German Version of the Palliative Care Outcome Scale. Journal of Pain Symptom Management. 2005;30(1):51–62. - PubMed
-
- Burnett P, Middleton W, Raphael B, Martinek N. Measuring Core Bereavement Phenomena. Psychological Medicine. 1997;27(1):49–57. - PubMed
-
- Carson MG, Fitch MI, Vachon ML. Measuring Patient Outcomes in Palliative Care: A Reliability and Validity Study of the Support Team Assessment Schedule. Palliative Medicine. 2000;14(1):25–36. - PubMed
-
- Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88(9):2164–71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources