Aging impacts transcriptomes but not genomes of hormone-dependent breast cancers
- PMID: 17850661
- PMCID: PMC2216076
- DOI: 10.1186/bcr1765
Aging impacts transcriptomes but not genomes of hormone-dependent breast cancers
Abstract
Introduction: Age is one of the most important risk factors for human malignancies, including breast cancer; in addition, age at diagnosis has been shown to be an independent indicator of breast cancer prognosis. Except for inherited forms of breast cancer, however, there is little genetic or epigenetic understanding of the biological basis linking aging with sporadic breast cancer incidence and its clinical behavior.
Methods: DNA and RNA samples from matched estrogen receptor (ER)-positive sporadic breast cancers diagnosed in either younger (age <or= 45 years) or older (age >or= 70 years) Caucasian women were analyzed by array comparative genomic hybridization and by expression microarrays. Array comparative genomic hybridization data were analyzed using hierarchical clustering and supervised age cohort comparisons. Expression microarray data were analyzed using hierarchical clustering and gene set enrichment analysis; differential gene expression was also determined by conditional permutation, and an age signature was derived using prediction analysis of microarrays.
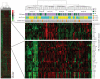
Results: Hierarchical clustering of genome-wide copy-number changes in 71 ER-positive DNA samples (27 younger women, 44 older women) demonstrated two age-independent genotypes; one with few genomic changes other than 1q gain/16q loss, and another with amplifications and low-level gains/losses. Age cohort comparisons showed no significant differences in total or site-specific genomic breaks and amplicon frequencies. Hierarchical clustering of 5.1 K genes variably expressed in 101 ER-positive RNA samples (53 younger women, 48 older women) identified six transcriptome subtypes with an apparent age bias (P < 0.05). Samples with higher expression of a poor outcome-associated proliferation signature were predominantly (65%) younger cases. Supervised analysis identified cancer-associated genes differentially expressed between the cohorts; with younger cases expressing more cell cycle genes and more than threefold higher levels of the growth factor amphiregulin (AREG), and with older cases expressing higher levels of four different homeobox (HOX) genes in addition to ER (ESR1). An age signature validated against two other independent breast cancer datasets proved to have >80% accuracy in discerning younger from older ER-positive breast cancer cases with characteristic differences in AREG and ESR1 expression.
Conclusion: These findings suggest that epigenetic transcriptome changes, more than genotypic variation, account for age-associated differences in sporadic breast cancer incidence and prognosis.
Figures



References
-
- Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, Thun M. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56:168–183. - PubMed
-
- DevCan – probability of developing or dying of cancer software http://www.srab.cancer.gov/devcan
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

