The multiple Maillard reactions of ribose and deoxyribose sugars and sugar phosphates
- PMID: 17850774
- PMCID: PMC2141680
- DOI: 10.1016/j.carres.2007.08.003
The multiple Maillard reactions of ribose and deoxyribose sugars and sugar phosphates
Abstract
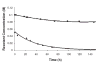
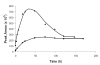
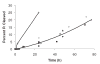
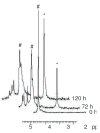
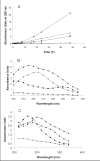
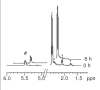
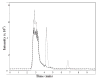
Ribose 5-phosphate (R5P) undergoes the Maillard reaction with amines at significantly higher rates than most other sugars and sugar phosphates. The presence of an intramolecular phosphate group, which catalyzes the early stages of the Maillard reaction, provides the opportunity for the R5P molecule to undergo novel reaction paths creating unique Maillard products. The initial set of reactions leading to an Amadori product (phosphorylated) and to an alpha-dicarbonyl phosphate compound follows a typical Maillard reaction sequence, but an observed phosphate hydrolysis accompanying the reaction adds to the complexity of the products formed. The reaction rate for the loss of R5P is partially dependent on the pK(a) of the amine but also is correlated to the protonation of an early intermediate of the reaction sequence. In the presence of oxygen, a carboxymethyl group conjugated to the amine is a major product of the reaction of R5P with N-acetyllysine while little of this product is generated in the absence of oxygen. Despite lacking a critical hydroxyl group necessary for the Maillard reaction, 2-deoxyribose 5-phosphate (dR5P) still generates an Amadori-like product (with a carbonyl on the C-3 carbon) and undergoes phosphate cleavage. Two highly UV-absorbing products of dR5P were amine derivatives of 5-methylene-2-pyrrolone and 2-formylpyrrole. The reaction of dR5P with certain amines generates a set of products that exhibit an interesting absorbance at 340nm and a high fluorescence.
Figures



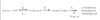
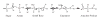
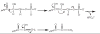

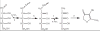
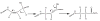
References
-
- Sandwick R, Johanson M, Breuer E. Ann N Y Acad Sci. 2005;1043:85–96. - PubMed
-
- Sharma SD, Pandey BN, Mishra KP, Sivakami S. J Biochem Mol Biol Biophys. 2002;6:233–242. - PubMed
-
- Farmer LJ, Hagan TDJ, Paraskevaws O. Role of Selected Precursors in Meat Flavor Formation. In: Xiong Y, Ho C-T, Shahidi F, editors. Quality Attributes of Muscle Foods. Kluwer Acad./Plenum Publisher; New York: 1999. pp. 159–172.
-
- Potman RP, van Wijk TA. ACS Symp Ser. Vol. 409. American Chemical Society; Washington: 1989. pp. 182–195.
-
- Rizzi GP. J Agric Food Chem. 2004;52:953–957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

