Complexes of 3d(n) metal ions with thiosemicarbazones: synthesis and antimicrobial activity
- PMID: 17851430
- PMCID: PMC6149331
- DOI: 10.3390/12040782
Complexes of 3d(n) metal ions with thiosemicarbazones: synthesis and antimicrobial activity
Abstract
The chelating behavior of the thiosemicarbazone derivatives of 2-hydroxy-8-R-tricyclo[7.3.1.0.(2,7)]tridecane-13-one (where R = H, CH3, C6H5) towards Co(II), Ni(II) and Cu(II) has been investigated by elemental analysis, molar conductivity measurements, UV-VIS, IR, ESR spectroscopy and thermal studies. It was deduced from the experiments performed that the ligands coordinate to metal ions in different ways--neutral bidentate or mononegative bidentate--depending on the nature of R. Also, if metal acetates are used instead of metal chlorides, the ligands coordinate in a mononegative bidentate fashion, regardless of the nature of R or the thiosemicarbazone type ligand. The antimicrobial activity of the ligands and of the complexes towards samples of Acinetobacter boumanii, Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa was determined.
Figures

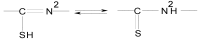

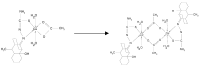

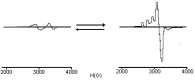
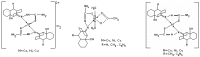
Similar articles
-
Synthesis, characterization antibacterial and antiproliferative activity of novel Cu(II) and Pd(II) complexes with 2-hydroxy-8-R-tricyclo[7.3.1.0.(2,7)]tridecane-13-one thiosemicarbazone.Eur J Med Chem. 2010 Apr;45(4):1627-34. doi: 10.1016/j.ejmech.2009.12.015. Epub 2010 Jan 22. Eur J Med Chem. 2010. PMID: 20096975
-
Spectroscopic and biological approach of Ni(II), Cu(II) and Co(II) complexes of 4-methoxy/ethoxybenzaldehyde thiosemicarbazone glyoxime.Spectrochim Acta A Mol Biomol Spectrosc. 2014;121:205-15. doi: 10.1016/j.saa.2013.10.040. Epub 2013 Oct 25. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24239764
-
Syntheses, spectroscopic characterization, thermal study, molecular modeling, and biological evaluation of novel Schiff's base benzil bis(5-amino-1,3,4-thiadiazole-2-thiol) with Ni(II), and Cu(II) metal complexes.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 25;137:749-60. doi: 10.1016/j.saa.2014.08.046. Epub 2014 Sep 3. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25262143
-
Antimicrobial and Structural Properties of Metal Ions Complexes with Thiosemicarbazide Motif and Related Heterocyclic Compounds.Curr Med Chem. 2019;26(4):664-693. doi: 10.2174/0929867325666180228164656. Curr Med Chem. 2019. PMID: 29493443 Review.
-
Metal transport across biomembranes: emerging models for a distinct chemistry.J Biol Chem. 2012 Apr 20;287(17):13510-7. doi: 10.1074/jbc.R111.319343. Epub 2012 Mar 2. J Biol Chem. 2012. PMID: 22389499 Free PMC article. Review.
Cited by
-
Spectral characterization and 3D molecular modeling studies of metal complexes involving the O, N-donor environment of quinazoline-4(3H)-one Schiff base and their biological studies.ScientificWorldJournal. 2014 Feb 11;2014:817365. doi: 10.1155/2014/817365. eCollection 2014. ScientificWorldJournal. 2014. PMID: 24678278 Free PMC article.
-
Synthesis and Characterization of New Palladium(II) Thiosemicarbazone Complexes and Their Cytotoxic Activity against Various Human Tumor Cell Lines.Bioinorg Chem Appl. 2013;2013:524701. doi: 10.1155/2013/524701. Epub 2013 Dec 11. Bioinorg Chem Appl. 2013. PMID: 24391528 Free PMC article.
-
Fragmentation Study, Dual Anti-Bactericidal and Anti-Viral Effects and Molecular Docking of Cobalt(III) Complexes.Int J Mol Sci. 2020 Nov 7;21(21):8355. doi: 10.3390/ijms21218355. Int J Mol Sci. 2020. PMID: 33171773 Free PMC article.
-
Synthesis, characterization, crystal structure and antimicrobial activity of copper(II) complexes with the Schiff base derived from 2-hydroxy-4-methoxybenzaldehyde.Molecules. 2015 Apr 2;20(4):5771-92. doi: 10.3390/molecules20045771. Molecules. 2015. PMID: 25849802 Free PMC article.
-
Microwave-assisted synthesis of new N₁,N₄-substituted thiosemicarbazones.Molecules. 2011 Dec 20;16(12):10668-84. doi: 10.3390/molecules161210668. Molecules. 2011. PMID: 22186954 Free PMC article.
References
-
- Collins F. M., Klayman D. L., Morrison N. E. Correlation between structure and anti-mycobacterial activity in a series of 2-acetylpyridine thiosemicarbazones. J. Gen. Microbiol. 1982;128:1349–1356. - PubMed
-
- Williams D. R. Metals, ligands and cancer. Chem. Rev. 1972;72:203–213. - PubMed
- John R. P., Sreekanth A., Rajakannan V., Ajith T. A., Kurup M. R. P. New copper(II) complexes thiosemicarbazones and polypyridyl co-ligands: structural, electrochemical of 2-hydroxyacetophenone N(4)-substituted and antimicrobial studies. Polyhedron. 2004;23:2549–2559.
- Belicchi-Ferrari M., Bisceglie F., Casoli C., Durot S., Morgenstern-Badarau I., Pelosi G., Pilotti E., Pinelli S., Tarasconi P. Copper(II) and Cobalt(III) Pyridoxal Thiosemicarbazone Complexes with Nitroprusside as Counterion: Syntheses, Electronic Properties, and Antileukemic Activity. J. Med. Chem. 2005;48(5):1671–1675. - PubMed
- Gulea A., Poirier D., Roy J., Spinu S., Coziri N., Birca M., Tapcov V. Anticancer effect of sulfanilamide complexes of Cu (II) with thiosemicarbazones of substituted salicylic aldehydes. COF-rRoCA 2004, Actes du Colloque Franco-Roumain de Chimie Applique, 3rd Bacau, Romania, Sept.22-26. 2004;68(Fr) Rom ISBN: 973-8392-36-5.
- Birca M., Tapcov V., Prisacari V., Gulea A., Buraciova S. Synthesis and antimicrobial properties of Cu complexes with thiosemicarbazones of substituted salicylic aldehydes. COFrRoCA 2004, Actes du Colloque Franco-Roumain de Chimie Applique, 3rdBacau, Romania, sept. 22-26. 2004;44-45(Fr) Rom ISBN: 973-8392-36-5.
-
- Kaska W. C., Carrans C., Michalowski J., Jackson J., Levinson W. Inhibition of the RNA dependent DNA polymerase and the malignant transforming ability of Rous sarcoma virus by Thiosemicarbazone-transition metal complexes. Bioinorg. Chem. 1978;8:245–254. - PubMed
-
- Klayman D. L., Bartosevich J. E., Griffith T. S., Mason C. J., Scovill J. P. 2-Acetylpyridine thiosemicarbazones. 1. A new class of potential antimalarial agents. J. Med. Chem. 1979;22:855–862. - PubMed
- Klayman D. L., Scovill J. P., Bartosevich J. P., Mason C. J. 2-Acetylpyridine thiosemicarbazones. 2. N4,N4-Disubstituted derivatives as potential antimalarial agents. J. Med. Chem. 1979;22:1367–1373. - PubMed
-
- Chan-Stier C. H., Minkel D. T., Petering D. H. Reactions of Bis(thiosemicarbazonato) Copper(II) complexes with tumor cells and mitochondria. Bioinorg. Chem. 1976;6:203–217. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

