Molecularly imprinted polymers for 5-fluorouracil release in biological fluids
- PMID: 17851432
- PMCID: PMC6149416
- DOI: 10.3390/12040805
Molecularly imprinted polymers for 5-fluorouracil release in biological fluids
Abstract
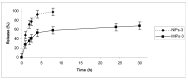
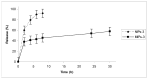
The aim of this work was to investigate the possibility of employing Molecularly Imprinted Polymers (MIPs) as a controlled release device for 5-fluorouracil (5-FU) in biological fluids, especially gastrointestinal ones, compared to Non Imprinted Polymers (NIPs). MIPs were synthesized using methacrylic acid (MAA) as functional monomer and ethylene glycol dimethacrylate (EGDMA) as crosslinking agent. The capacity of the polymer to recognize and to bind the template selectively in both organic and aqueous media was evaluated. An in vitro release study was performed both in gastrointestinal and in plasma simulating fluids. The imprinted polymers bound much more 5-Fu than the corresponding non-imprinted ones and showed a controlled/sustained drug release, with MIPs release rate being indeed much more sustained than that obtained from NIPs. These polymers represent a potential valid system for drug delivery and this study indicates that the selective binding characteristic of molecularly imprinted polymers is promising for the preparation of novel controlled release drug dosage form.
Figures

References
-
- Caro E., Marce R.M., Cormack P.A.G., Sherrington D.C., Borrull F. On-line solid-phase extraction with molecularly imprinted polymers to selectively extract substituted 4-chlorophenols and 4-nitrophenol from water. J. Chromatogr. A. 2003;995:233–238. doi: 10.1016/S0021-9673(03)00543-0. - DOI - PubMed
-
- Spizzirri U.G., Peppas N.A. Structural analysis and diffusional behavior of molecularly imprinted polymer networks for cholesterol recognition. Chem. Mat. 2005;17:6719–6727. doi: 10.1021/cm0478531. - DOI
-
- Puoci F., Iemma F., Cirillo G., Trombino S., Cassano R., Picci N. Molecularly Imprinted polymers for selective adsorption of cholesterol from aqueous environment. E-polymers. 2007;13:1–9.
-
- Hishiya T., Asanuma H., Komiyama M. Molecularly imprinted cyclodextrin polymers as stationary phases of high performance liquid chromatography. Polym. J. 2003;35:440–445. doi: 10.1295/polymj.35.440. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

