TLR4 activation mediates kidney ischemia/reperfusion injury
- PMID: 17853945
- PMCID: PMC1974864
- DOI: 10.1172/JCI31008
TLR4 activation mediates kidney ischemia/reperfusion injury
Abstract
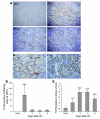
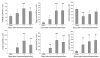
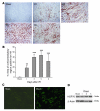
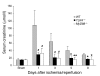
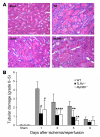
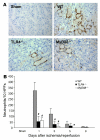
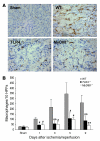
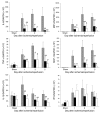
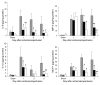
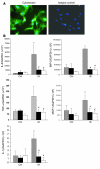
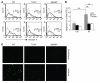
Ischemia/reperfusion injury (IRI) may activate innate immunity through the engagement of TLRs by endogenous ligands. TLR4 expressed within the kidney is a potential mediator of innate activation and inflammation. Using a mouse model of kidney IRI, we demonstrated a significant increase in TLR4 expression by tubular epithelial cells (TECs) and infiltrating leukocytes within the kidney following ischemia. TLR4 signaling through the MyD88-dependent pathway was required for the full development of kidney IRI, as both TLR4(-/-) and MyD88(-/-) mice were protected against kidney dysfunction, tubular damage, neutrophil and macrophage accumulation, and expression of proinflammatory cytokines and chemokines. In vitro, WT kidney TECs produced proinflammatory cytokines and chemokines and underwent apoptosis after ischemia. These effects were attenuated in TLR4(-/-) and MyD88(-/-) TECs. In addition, we demonstrated upregulation of the endogenous ligands high-mobility group box 1 (HMGB1), hyaluronan, and biglycan, providing circumstantial evidence that one or more of these ligands may be the source of TLR4 activation. To determine the relative contribution of TLR4 expression by parenchymal cells or leukocytes to kidney damage during IRI, we generated chimeric mice. TLR4(-/-) mice engrafted with WT hematopoietic cells had significantly lower serum creatinine and less tubular damage than WT mice reconstituted with TLR4(-/-) BM, suggesting that TLR4 signaling in intrinsic kidney cells plays the dominant role in mediating kidney damage.
Figures
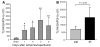
References
-
- Tilney N.L., Guttmann R.D. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation. 1997;64:945–947. - PubMed
-
- Chapman J.R., O’Connell P.J., Nankivell B.J. Chronic renal allograft dysfunction. J. Am. Soc. Nephrol. 2005;16:3015–3026. - PubMed
-
- Bonventre J.V., Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. - PubMed
-
- Jo S.K., Sung S.A., Cho W.Y., Go K.J., Kim H.K. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol. Dial. Transplant. 2006;21:1231–1239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

