Adherens and tight junctions: structure, function and connections to the actin cytoskeleton
- PMID: 17854762
- PMCID: PMC2682436
- DOI: 10.1016/j.bbamem.2007.07.012
Adherens and tight junctions: structure, function and connections to the actin cytoskeleton
Abstract
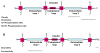
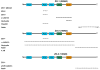
Adherens junctions and Tight junctions comprise two modes of cell-cell adhesion that provide different functions. Both junctional complexes are proposed to associate with the actin cytoskeleton, and formation and maturation of cell-cell contacts involves reorganization of the actin cytoskeleton. Adherens junctions initiate cell-cell contacts, and mediate the maturation and maintenance of the contact. Adherens junctions consist of the transmembrane protein E-cadherin, and intracellular components, p120-catenin, beta-catenin and alpha-catenin. Tight junctions regulate the paracellular pathway for the movement of ions and solutes in-between cells. Tight junctions consist of the transmembrane proteins occludin and claudin, and the cytoplasmic scaffolding proteins ZO-1, -2, and -3. This review discusses the binding interactions of the most studied proteins that occur within each of these two junctional complexes and possible modes of regulation of these interactions, and the different mechanisms that connect and regulate interactions with the actin cytoskeleton.
Figures
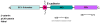

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

