Objectively monitored patching regimens for treatment of amblyopia: randomised trial
- PMID: 17855283
- PMCID: PMC2001048
- DOI: 10.1136/bmj.39301.460150.55
Objectively monitored patching regimens for treatment of amblyopia: randomised trial
Abstract
Objectives: To compare visual outcome in response to two prescribed rates of occlusion (six hours a day and 12 hours a day).
Design: Unmasked randomised trial.
Setting: Research clinics in two London hospitals.
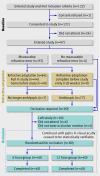
Participants: 97 children with a confirmed diagnosis of amblyopia associated with strabismus, anisometropia, or both.
Interventions: 18 week period of wearing glasses (refractive adaptation) followed by occlusion prescribed ("patching") for six or 12 hours a day.
Main outcome measures: Visual acuity measured by logMAR letter recognition; objectively monitored rate of occlusion (hours a day).
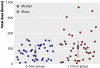
Results: The mean age of children at study entry was 5.6 (SD 1.5) years. Ninety were eligible for occlusion but 10 dropped out in this phase, leaving 80 children who were randomised to a prescribed dose rate of six (n=40) or 12 (n=40) hours a day. The mean change in visual acuity of the amblyopic eye was not significantly different (P=0.64) between the two groups (0.26 (95% confidence interval 0.21 to 0.31) log units in six hour group; 0.24 (0.19 to 0.29) log units in 12 hour group). The mean dose rates (hours a day) actually received, however, were also not significantly different (4.2 (3.7 to 4.7) in six hour group v 6.2 (5.1 to 7.3) in 12 hour group; P=0.06). The visual outcome was similar for those children who received three to six hours a day or more than six to 12 hours a day, but significantly better than that in children who received less than three hours a day. Children aged under 4 required significantly less occlusion than older children. Visual outcome was not influenced by type of amblyopia.
Conclusions: Substantial (six hours a day) and maximal (12 hours a day) prescribed occlusion results in similar visual outcome. On average, the occlusion dose received in the maximal group was only 50% more than in the substantial group and in both groups was much less than that prescribed. Younger children required the least occlusion.
Trials registration: Clinical Trials NCT00274664.
Conflict of interest statement
Competing interests: None declared.
Figures



Comment in
-
Occlusion therapy for amblyopia.BMJ. 2007 Oct 6;335(7622):678-9. doi: 10.1136/bmj.39343.640938.80. BMJ. 2007. PMID: 17916815 Free PMC article.
-
Occlusion studies are ambiguous.BMJ. 2007 Oct 27;335(7625):842. doi: 10.1136/bmj.39374.474745.BE. BMJ. 2007. PMID: 17962255 Free PMC article. No abstract available.
References
-
- Daw NW. Critical periods in the visual system. In: Hopkins B, Johnson SP, eds. Neurobiology of infant vision Westport, CT: Praeger, 2003:43-103.
-
- Stewart CE, Moseley MJ, Stephens DA, Fielder AR, the MOTAS cooperative. The treatment dose-response in amblyopia therapy: results from the monitored occlusion treatment of amblyopia study (MOTAS) Invest Ophthalmol Vis Sci 2004;45:3048-54. - PubMed
-
- Rahi J, Logan S, Timms C, Russell-Eggitt I, Taylor D. Risk, causes and outcomes of visual impairment after loss of vision in the non-amblyopic eye: a population-based study. Lancet 2002;360:597-602. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
