A critical three-way junction is conserved in budding yeast and vertebrate telomerase RNAs
- PMID: 17855392
- PMCID: PMC2094081
- DOI: 10.1093/nar/gkm713
A critical three-way junction is conserved in budding yeast and vertebrate telomerase RNAs
Abstract
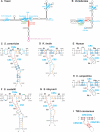
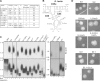
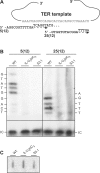
The telomerase ribonucleoprotein copies a short template within its integral RNA moiety onto eukaryotic chromosome ends, compensating for incomplete replication and degradation. Non-template regions of telomerase RNA (TER) are also crucial for telomerase function, yet they are highly divergent in sequence among species and their roles are largely unclear. Using both phylogenetic and mutational analyses, we predicted secondary structures for TERs from Kluyveromyces budding yeast species. A comparison of these secondary structure models with the published model for the Saccharomyces cerevisiae TER reveals a common arrangement into three long arms, a templating domain in the center and several conserved elements in the same positions within the structure. One of them, a three-way junction element, is highly conserved in budding yeast TERs. This element also shows sequence and structure similarity to the critical CR4-CR5 activating domain of vertebrate TERs. Mutational analysis in Kluyveromyces lactis confirmed that this element, and in particular the residues conserved across yeast and vertebrates, is critical for telomerase action both in vivo and in vitro. These findings demonstrate that despite the extreme divergence of TER sequences from different organisms, they do share conserved elements, which presumably carry out common roles in telomerase function.
Figures

References
-
- Bertuch AA, Lundblad V. The maintenance and masking of chromosome termini. Curr. Opin. Cell. Biol. 2006;18:247–253. - PubMed
-
- Autexier C, Lue NF. The structure and function of telomerase reverse transcriptase. Annu. Rev. Biochem. 2006;75:493–517. - PubMed
-
- Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 2004;73:177–208. - PubMed
-
- Romero DP, Blackburn EH. A conserved secondary structure for telomerase RNA. Cell. 1991;67:343–353. - PubMed
-
- Lingner J, Hendrick LL, Cech TR. Telomerase RNAs of different ciliates have a common secondary structure and a permuted template. Genes Dev. 1994;8:1984–1998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

