Intestinal hyperplasia induced by simian virus 40 large tumor antigen requires E2F2
- PMID: 17855529
- PMCID: PMC2169091
- DOI: 10.1128/JVI.01658-07
Intestinal hyperplasia induced by simian virus 40 large tumor antigen requires E2F2
Abstract
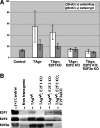
The simian virus 40 large T antigen contributes to neoplastic transformation, in part, by targeting the Rb family of tumor suppressors. There are three known Rb proteins, pRb, p130, and p107, all of which block the cell cycle by preventing the transcription of genes regulated by the E2F family of transcription factors. T antigen interacts directly with Rb proteins and disrupts Rb-E2F complexes both in vitro and in cultured cells. Consequently, T antigen is thought to inhibit transcriptional repression by the Rb family proteins by disrupting their interaction with E2F proteins, thus allowing E2F-dependent transcription and the expression of cellular genes needed for entry into S phase. This model predicts that active E2F-dependent transcription is required for T-antigen-induced transformation. To test this hypothesis, we have examined the status of Rb-E2F complexes in murine enterocytes. Previous studies have shown that T antigen drives enterocytes into S phase, resulting in intestinal hyperplasia, and that the induction of enterocyte proliferation requires T-antigen binding to Rb proteins. In this paper, we show that normal growth-arrested enterocytes contain p130-E2F4 complexes and that T-antigen expression destroys these complexes, most likely by stimulating p130 degradation. Furthermore, unlike their normal counterparts, enterocytes expressing T antigen contain abundant levels of E2F2 and E2F3a. Concomitantly, T-antigen-induced intestinal proliferation is reduced in mice lacking either E2F2 alone or both E2F2 and E2F3a, but not in mice lacking E2F1. These studies support a model in which T antigen eliminates Rb-E2F repressive complexes so that specific activator E2Fs can drive S-phase entry.
Figures

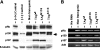



Similar articles
-
Enterocyte proliferation and intestinal hyperplasia induced by simian virus 40 T antigen require a functional J domain.J Virol. 2007 Sep;81(17):9481-9. doi: 10.1128/JVI.00922-07. Epub 2007 Jun 20. J Virol. 2007. PMID: 17581980 Free PMC article.
-
Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation.Mol Cell Biol. 1995 Oct;15(10):5800-10. doi: 10.1128/MCB.15.10.5800. Mol Cell Biol. 1995. PMID: 7565733 Free PMC article.
-
A complex between E2F and the pRb-related protein p130 is specifically targeted by the simian virus 40 large T antigen during cell transformation.Oncogene. 1995 Jun 1;10(11):2067-78. Oncogene. 1995. PMID: 7784051
-
Role of the interaction between large T antigen and Rb family members in the oncogenicity of JC virus.Oncogene. 2006 Aug 28;25(38):5294-301. doi: 10.1038/sj.onc.1209681. Oncogene. 2006. PMID: 16936750 Review.
-
TGFbeta-mediated formation of pRb-E2F complexes in human myeloid leukemia cells.Biochem Biophys Res Commun. 2008 May 2;369(2):277-80. doi: 10.1016/j.bbrc.2008.02.051. Epub 2008 Feb 21. Biochem Biophys Res Commun. 2008. PMID: 18294958 Review.
Cited by
-
Emerging roles of E2Fs in cancer: an exit from cell cycle control.Nat Rev Cancer. 2009 Nov;9(11):785-97. doi: 10.1038/nrc2696. Nat Rev Cancer. 2009. PMID: 19851314 Free PMC article. Review.
-
Cell-type specific regulation of gene expression by simian virus 40 T antigens.Virology. 2009 Mar 30;386(1):183-91. doi: 10.1016/j.virol.2008.12.038. Epub 2009 Feb 8. Virology. 2009. PMID: 19201438 Free PMC article.
-
Induction of interferon-stimulated genes by Simian virus 40 T antigens.Virology. 2010 Oct 25;406(2):202-11. doi: 10.1016/j.virol.2010.07.018. Epub 2010 Aug 7. Virology. 2010. PMID: 20692676 Free PMC article.
-
Viral oncogene expression in the stem/progenitor cell compartment of the mouse intestine induces adenomatous polyps.Mol Cancer Res. 2014 Oct;12(10):1355-64. doi: 10.1158/1541-7786.MCR-14-0166. Epub 2014 Jul 3. Mol Cancer Res. 2014. PMID: 24994749 Free PMC article.
-
E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells.Nature. 2009 Dec 17;462(7275):930-4. doi: 10.1038/nature08677. Nature. 2009. PMID: 20016602 Free PMC article.
References
-
- Ahuja, D., M. T. Saenz-Robles, and J. M. Pipas. 2005. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 24:7729-7745. - PubMed
-
- Athanasiou, M. C., W. Yunis, N. Coleman, R. Ehlenfeldt, H. B. Clark, H. T. Orr, and R. M. Feddersen. 1998. The transcription factor E2F-1 in SV40 T antigen-induced cerebellar Purkinje cell degeneration. Mol. Cell. Neurosci. 12:16-28. - PubMed
-
- Blais, A., and B. D. Dynlacht. 2004. Hitting their targets: an emerging picture of E2F and cell cycle control. Curr. Opin. Genet. Dev. 14:527-532. - PubMed
-
- Chandrasekaran, C., C. M. Coopersmith, and J. I. Gordon. 1996. Use of normal and transgenic mice to examine the relationship between terminal differentiation of intestinal epithelial cells and accumulation of their cell cycle regulators. J. Biol. Chem. 271:28414-28421. - PubMed
-
- Chen, J., G. J. Tobin, J. M. Pipas, and T. Van Dyke. 1992. T-antigen mutant activities in vivo: roles of p53 and pRB binding in tumorigenesis of the choroid plexus. Oncogene 7:1167-1175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HD047470/HD/NICHD NIH HHS/United States
- P01 CA097189/CA/NCI NIH HHS/United States
- R01 CA085619/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- DK61218/DK/NIDDK NIH HHS/United States
- R01 CA082259/CA/NCI NIH HHS/United States
- R01CA85619/CA/NCI NIH HHS/United States
- R01 DK061218/DK/NIDDK NIH HHS/United States
- R01 CA098956/CA/NCI NIH HHS/United States
- P01CA097189/CA/NCI NIH HHS/United States
- R02CA82259/CA/NCI NIH HHS/United States
- CA098956/CA/NCI NIH HHS/United States
- DK058404/DK/NIDDK NIH HHS/United States
- R01 HD047470/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

