Suppression of viremia and evolution of human immunodeficiency virus type 1 drug resistance in a macaque model for antiretroviral therapy
- PMID: 17855539
- PMCID: PMC2169021
- DOI: 10.1128/JVI.01301-07
Suppression of viremia and evolution of human immunodeficiency virus type 1 drug resistance in a macaque model for antiretroviral therapy
Abstract
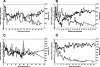
Antiretroviral therapy (ART) in human immunodeficiency virus type 1 (HIV-1)-infected patients does not clear the infection and can select for drug resistance over time. Not only is drug-resistant HIV-1 a concern for infected individuals on continual therapy, but it is an emerging problem in resource-limited settings where, in efforts to stem mother-to-child-transmission of HIV-1, transient nonnucleoside reverse transcriptase inhibitor (NNRTI) therapy given during labor can select for NNRTI resistance in both mother and child. Questions of HIV-1 persistence and drug resistance are highly amenable to exploration within animals models, where therapy manipulation is less constrained. We examined a pigtail macaque infection model responsive to anti-HIV-1 therapy to study the development of resistance. Pigtail macaques were infected with a pathogenic simian immunodeficiency virus encoding HIV-1 reverse transcriptase (RT-SHIV) to examine the impact of prior exposure to a NNRTI on subsequent ART comprised of a NNRTI and two nucleoside RT inhibitors. K103N resistance-conferring mutations in RT rapidly accumulated in 2/3 infected animals after NNRTI monotherapy and contributed to virologic failure during ART in 1/3 animals. By contrast, ART effectively suppressed RT-SHIV in 5/6 animals. These data indicate that suboptimal therapy facilitates HIV-1 drug resistance and suggest that this model can be used to investigate persisting viral reservoirs.
Figures




References
-
- Akiyama, H., E. Ido, W. Akahata, T. Kuwata, T. Miura, and M. Hayami. 2003. Construction and in vivo infection of a new simian/human immunodeficiency virus chimera containing the reverse transcriptase gene and the 3′ half of the genomic region of human immunodeficiency virus type 1. J. Gen. Virol. 84:1663-1669. - PubMed
-
- Ambrose, Z., V. Boltz, S. Palmer, J. M. Coffin, S. H. Hughes, and V. N. Kewalramani. 2004. In vitro characterization of a simian immunodeficiency virus-human immunodeficiency virus (HIV) chimera expressing HIV type 1 reverse transcriptase to study antiviral resistance in pigtail macaques. J. Virol. 78:13553-13561. - PMC - PubMed
-
- Arnedo-Valero, M., F. Garcia, C. Gil, T. Guila, E. Fumero, P. Castro, J. L. Blanco, J. M. Miro, T. Pumarola, and J. M. Gatell. 2005. Risk of selecting de novo drug-resistance mutations during structured treatment interruptions in patients with chronic HIV infection. Clin. Infect. Dis. 41:883-890. - PubMed
-
- Ayouba, A., G. Tene, P. Cunin, Y. Foupouapouognigni, E. Menu, A. Kfutwah, J. Thonnon, G. Scarlatti, M. Monny-Lobe, N. Eteki, C. Kouanfack, M. Tardy, R. Leke, M. Nkam, A. E. Nlend, F. Barre-Sinoussi, P. M. Martin, and E. Nerrienet. 2003. Low rate of mother-to-child transmission of HIV-1 after nevirapine intervention in a pilot public health program in Yaounde, Cameroon. J. Acquir. Immune Defic. Syndr. 34:274-280. - PubMed
-
- Bailey, J. R., A. R. Sedaghat, T. Kieffer, T. Brennan, P. K. Lee, M. Wind-Rotolo, C. M. Haggerty, A. R. Kamireddi, Y. Liu, J. Lee, D. Persaud, J. E. Gallant, J. Cofrancesco, Jr., T. C. Quinn, C. O. Wilke, S. C. Ray, J. D. Siliciano, R. E. Nettles, and R. F. Siliciano. 2006. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol. 80:6441-6457. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

