Influenza virus mRNA translation revisited: is the eIF4E cap-binding factor required for viral mRNA translation?
- PMID: 17855553
- PMCID: PMC2168979
- DOI: 10.1128/JVI.01105-07
Influenza virus mRNA translation revisited: is the eIF4E cap-binding factor required for viral mRNA translation?
Abstract
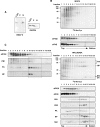
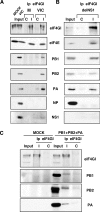
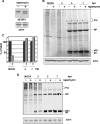
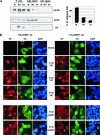
Influenza virus mRNAs bear a short capped oligonucleotide sequence at their 5' ends derived from the host cell pre-mRNAs by a "cap-snatching" mechanism, followed immediately by a common viral sequence. At their 3' ends, they contain a poly(A) tail. Although cellular and viral mRNAs are structurally similar, influenza virus promotes the selective translation of its mRNAs despite the inhibition of host cell protein synthesis. The viral polymerase performs the cap snatching and binds selectively to the 5' common viral sequence. As viral mRNAs are recognized by their own cap-binding complex, we tested whether viral mRNA translation occurs without the contribution of the eIF4E protein, the cellular factor required for cap-dependent translation. Here, we show that influenza virus infection proceeds normally in different situations of functional impairment of the eIF4E factor. In addition, influenza virus polymerase binds to translation preinitiation complexes, and furthermore, under conditions of decreased eIF4GI association to cap structures, an increase in eIF4GI binding to these structures was found upon influenza virus infection. This is the first report providing evidence that influenza virus mRNA translation proceeds independently of a fully active translation initiation factor (eIF4E). The data reported are in agreement with a role of viral polymerase as a substitute for the eIF4E factor for viral mRNA translation.
Figures


References
-
- Beloso, A., C. Martínez, J. Valcárcel, J. Fernández-Santarén, and J. Ortín. 1992. Degradation of cellular mRNA during influenza virus infection: its possible role in protein synthesis shutoff. J. Gen. Virol. 73:575-581. - PubMed
-
- Burgui, I., T. Aragón, J. Ortín, and A. Nieto. 2003. PABP1 and eIF4GI associate to influenza virus NS1 protein in viral mRNA translation initiation complexes. J. Virol. 84:3263-3274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

