Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy
- PMID: 17855601
- PMCID: PMC6672644
- DOI: 10.1523/JNEUROSCI.2761-07.2007
Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy
Abstract
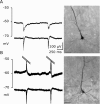
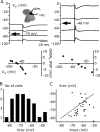
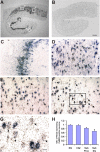
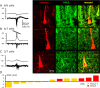
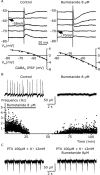
Changes in chloride (Cl-) homeostasis may be involved in the generation of some epileptic activities. In this study, we asked whether Cl- homeostasis, and thus GABAergic signaling, is altered in tissue from patients with mesial temporal lobe epilepsy associated with hippocampal sclerosis. Slices prepared from this human tissue generated a spontaneous interictal-like activity that was initiated in the subiculum. Records from a minority of subicular pyramidal cells revealed depolarizing GABA(A) receptor-mediated postsynaptic events, indicating a perturbed Cl- homeostasis. We assessed possible contributions of changes in expression of the potassium-chloride cotransporter KCC2. Double in situ hybridization showed that mRNA for KCC2 was absent from approximately 30% of CaMKIIalpha (calcium/calmodulin-dependent protein kinase IIalpha)-positive subicular pyramidal cells. Combining intracellular recordings with biocytin-filled electrodes and KCC2 immunochemistry, we observed that all cells that were hyperpolarized during interictal events were immunopositive for KCC2, whereas the majority of depolarized cells were immunonegative. Bumetanide, at doses that selectively block the chloride-importing potassium-sodium-chloride cotransporter NKCC1, produced a hyperpolarizing shift in GABA(A) reversal potentials and suppressed interictal activity. Changes in Cl- transporter expression thus contribute to human epileptiform activity, and molecules acting on these transporters may be useful antiepileptic drugs.
Figures

Comment in
-
Altered neuronal chloride homeostasis and excitatory GABAergic signaling in human temporal lobe epilepsy.Epilepsy Curr. 2008 Mar-Apr;8(2):51-3. doi: 10.1111/j.1535-7511.2008.00235.x. Epilepsy Curr. 2008. PMID: 18330470 Free PMC article. No abstract available.
Similar articles
-
The epileptic human hippocampal cornu ammonis 2 region generates spontaneous interictal-like activity in vitro.Brain. 2009 Nov;132(Pt 11):3032-46. doi: 10.1093/brain/awp238. Epub 2009 Sep 18. Brain. 2009. PMID: 19767413
-
Cation-chloride cotransporters and GABA-ergic innervation in the human epileptic hippocampus.Epilepsia. 2007 Apr;48(4):663-73. doi: 10.1111/j.1528-1167.2007.00986.x. Epub 2007 Feb 23. Epilepsia. 2007. PMID: 17319917
-
Anomalous levels of Cl- transporters cause a decrease of GABAergic inhibition in human peritumoral epileptic cortex.Epilepsia. 2011 Sep;52(9):1635-44. doi: 10.1111/j.1528-1167.2011.03111.x. Epub 2011 Jun 2. Epilepsia. 2011. PMID: 21635237
-
Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII.J Physiol. 2005 Jan 1;562(Pt 1):27-36. doi: 10.1113/jphysiol.2004.077495. Epub 2004 Nov 4. J Physiol. 2005. PMID: 15528236 Free PMC article. Review.
-
[Epileptiform activities generated in vitro by human temporal lobe tissue].Neurochirurgie. 2008 May;54(3):148-58. doi: 10.1016/j.neuchi.2008.02.004. Epub 2008 Apr 16. Neurochirurgie. 2008. PMID: 18420229 Review. French.
Cited by
-
GABAA signaling, focal epileptiform synchronization and epileptogenesis.Front Neural Circuits. 2022 Oct 5;16:984802. doi: 10.3389/fncir.2022.984802. eCollection 2022. Front Neural Circuits. 2022. PMID: 36275847 Free PMC article.
-
Ketogenic Diet Provided During Three Months Increases KCC2 Expression but Not NKCC1 in the Rat Dentate Gyrus.Front Neurosci. 2020 Jul 7;14:673. doi: 10.3389/fnins.2020.00673. eCollection 2020. Front Neurosci. 2020. PMID: 32733191 Free PMC article.
-
Neuroinflammation, neuroautoimmunity, and the co-morbidities of complex regional pain syndrome.J Neuroimmune Pharmacol. 2013 Jun;8(3):452-69. doi: 10.1007/s11481-012-9392-x. Epub 2012 Aug 25. J Neuroimmune Pharmacol. 2013. PMID: 22923151 Free PMC article. Review.
-
A single seizure episode leads to rapid functional activation of KCC2 in the neonatal rat hippocampus.J Neurosci. 2010 Sep 8;30(36):12028-35. doi: 10.1523/JNEUROSCI.3154-10.2010. J Neurosci. 2010. PMID: 20826666 Free PMC article.
-
Cell Volume Control in Healthy Brain and Neuropathologies.Curr Top Membr. 2018;81:385-455. doi: 10.1016/bs.ctm.2018.07.006. Epub 2018 Aug 27. Curr Top Membr. 2018. PMID: 30243438 Free PMC article. Review.
References
-
- Amaral D, Insausti R. The hippocampal formation. In: Paxinos G, editor. The human nervous system. San Diego: Academic; 1990. pp. 711–755.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
