Bimodal action of menthol on the transient receptor potential channel TRPA1
- PMID: 17855602
- PMCID: PMC6672629
- DOI: 10.1523/JNEUROSCI.2221-07.2007
Bimodal action of menthol on the transient receptor potential channel TRPA1
Abstract
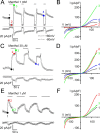
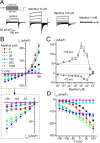
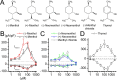
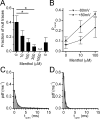
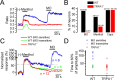
TRPA1 is a calcium-permeable nonselective cation transient receptor potential (TRP) channel that functions as an excitatory ionotropic receptor in nociceptive neurons. TRPA1 is robustly activated by pungent substances in mustard oil, cinnamon, and garlic and mediates the inflammatory actions of environmental irritants and proalgesic agents. Here, we demonstrate a bimodal sensitivity of TRPA1 to menthol, a widely used cooling agent and known activator of the related cold receptor TRPM8. In whole-cell and single-channel recordings of heterologously expressed TRPA1, submicromolar to low-micromolar concentrations of menthol cause channel activation, whereas higher concentrations lead to a reversible channel block. In addition, we provide evidence for TRPA1-mediated menthol responses in mustard oil-sensitive trigeminal ganglion neurons. Our data indicate that TRPA1 is a highly sensitive menthol receptor that very likely contributes to the diverse psychophysical sensations after topical application of menthol to the skin or mucous membranes of the oral and nasal cavities.
Figures



References
-
- Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, Matsumura K, Kobayashi S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res. 2005;136:91–98. - PubMed
-
- Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–2282. - PubMed
-
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. - PubMed
-
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
