A role for the MLL fusion partner ENL in transcriptional elongation and chromatin modification
- PMID: 17855633
- PMCID: PMC2234781
- DOI: 10.1182/blood-2007-05-090514
A role for the MLL fusion partner ENL in transcriptional elongation and chromatin modification
Abstract
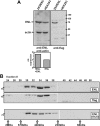
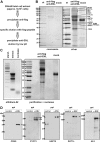
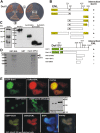
Chimeric proteins joining the histone methyltransferase MLL with various fusion partners trigger distinctive lymphoid and myeloid leukemias. Here, we immunopurified proteins associated with ENL, a protein commonly fused to MLL. Identification of these ENL-associated proteins (EAPs) by mass spectrometry revealed enzymes with a known role in transcriptional elongation (RNA polymerase II C-terminal domain kinase [RNAPolII CTD] positive transcription elongation factor b [pTEFb]), and in chromatin modification (histone-H3 methyltransferase DOT1L) as well as other frequent MLL partners (AF4, AF5q31, and LAF4), and polycomb group members (RING1, CBX8, and BCoR). The composition of EAP was further verified by coimmunoprecipitation, 2-hybrid analysis, pull-down, and colocalization experiments. Purified EAP showed a histone H3 lysine 79-specific methylase activity, displayed a robust RNAPolII CTD kinase function, and counteracted the effect of the pTEFb inhibitor 5,6-dichloro-benzimidazole-riboside. In vivo, an ENL knock-down diminished genome-wide as well as gene-specific H3K79 dimethylation, reduced global run-on elongation, and inhibited transient transcriptional reporter activity. According to structure-function data, DOT1L recruitment was important for transformation by the MLL-ENL fusion derivative. These results suggest a function of ENL in histone modification and transcriptional elongation.
Figures



References
-
- Gilliland DG, Jordan CT, Felix CA. The molecular basis of leukemia. Hematology Am Soc Hematol Educ Program. 2004:80–97. - PubMed
-
- Hess JL. Mechanisms of transformation by MLL. Crit Rev Eukaryot Gene Expr. 2004;14:235–254. - PubMed
-
- Slany RK. When epigenetics kills: MLL fusion proteins in leukemia [review]. Hematol Oncol. 2005;23:1–9. - PubMed
-
- Schreiner SA, Garcia-Cuellar MP, Fey GH, Slany RK. The leukemogenic fusion of MLL with ENL creates a novel transcriptional transactivator. Leukemia. 1999;13:1525–1533. - PubMed
-
- So CW, Cleary ML. Common mechanism for oncogenic activation of MLL by forkhead family proteins. Blood. 2003;101:633–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

