Disuse of rat muscle in vivo reduces protein kinase C activity controlling the sarcolemma chloride conductance
- PMID: 17855757
- PMCID: PMC2276996
- DOI: 10.1113/jphysiol.2007.141358
Disuse of rat muscle in vivo reduces protein kinase C activity controlling the sarcolemma chloride conductance
Abstract
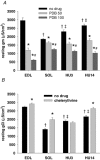
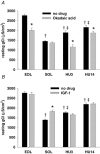
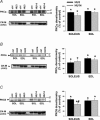
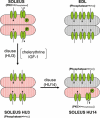
Muscle disuse produced by hindlimb unloading (HU) induces severe atrophy and slow-to-fast fibre type transition of the slow-twitch soleus muscle (Sol). After 2 weeks HU, the resting ClC-1 chloride conductance (g(Cl)) of sarcolemma, which controls muscle excitability, increases in Sol toward a value typical of the fast-twitch EDL muscle. After 3 days of HU, the g(Cl) increases as well before initiation of fibre type transition. Since ClC-1 channels are acutely silenced by PKC-dependent phosphorylation, we studied the modulation of g(Cl) by PKC and serine-threonine phosphatase in Sol during HU, using a number of pharmacological tools. We show that a fraction of ClC-1 channels of control Sol are maintained in an inactive state by PKC basal activity, which contributes to the lower g(Cl) in control Sol compared to EDL. After 14 days of HU, PKC/phosphatase manipulation produces effects on Sol g(Cl) that corroborate the partial slow-to-fast transition. After 3 days of HU, the early increase of g(Cl) in Sol is entirely attributable to a reduction of PKC activity and/or activation of phosphatase, maintaining ClC-1 channels in a fully active state. Accordingly, we found that HU reduces expression of PKCalpha, epsilon, and isoenzymes in Sol and EDL muscles and reduces total PKC activity. Moreover, we show that the rheobase current is increased in Sol muscle fibres as soon as after 3 days of HU, most probably in relation to the increased g(Cl). In conclusion, Sol muscle disuse is characterized by a rapid reduction of PKC activity, which reduces muscle excitability and is likely to contribute to disuse-induced muscle impairment.
Figures

Similar articles
-
Change of chloride ion channel conductance is an early event of slow-to-fast fibre type transition during unloading-induced muscle disuse.Brain. 2002 Jul;125(Pt 7):1510-21. doi: 10.1093/brain/awf162. Brain. 2002. PMID: 12077001
-
Antioxidant treatment of hindlimb-unloaded mouse counteracts fiber type transition but not atrophy of disused muscles.Pharmacol Res. 2010 Jun;61(6):553-63. doi: 10.1016/j.phrs.2010.01.012. Epub 2010 Jan 29. Pharmacol Res. 2010. PMID: 20116431
-
Potential targets for skeletal muscle impairment by hypogravity: basic characterization of resting ionic conductances and mechanical threshold of rat fast- and slow-twitch muscle fibers.J Gravit Physiol. 1998 Jul;5(1):P75-6. J Gravit Physiol. 1998. PMID: 11542372
-
Recovery of the soleus muscle after short- and long-term disuse induced by hindlimb unloading: effects on the electrical properties and myosin heavy chain profile.Neurobiol Dis. 2005 Mar;18(2):356-65. doi: 10.1016/j.nbd.2004.09.016. Neurobiol Dis. 2005. PMID: 15686964
-
Early postdenervation depolarization is controlled by acetylcholine and glutamate via nitric oxide regulation of the chloride transporter.Neurochem Res. 2003 Apr;28(3-4):575-85. doi: 10.1023/a:1022833709448. Neurochem Res. 2003. PMID: 12675147 Review.
Cited by
-
Adaptation of mouse skeletal muscle to long-term microgravity in the MDS mission.PLoS One. 2012;7(3):e33232. doi: 10.1371/journal.pone.0033232. Epub 2012 Mar 28. PLoS One. 2012. PMID: 22470446 Free PMC article.
-
Sarcolemmal ATP-sensitive potassium channels modulate skeletal muscle function under low-intensity workloads.J Gen Physiol. 2014 Jan;143(1):119-34. doi: 10.1085/jgp.201311063. Epub 2013 Dec 16. J Gen Physiol. 2014. PMID: 24344248 Free PMC article.
-
Angiotensin II modulates mouse skeletal muscle resting conductance to chloride and potassium ions and calcium homeostasis via the AT1 receptor and NADPH oxidase.Am J Physiol Cell Physiol. 2014 Oct 1;307(7):C634-47. doi: 10.1152/ajpcell.00372.2013. Epub 2014 Jul 30. Am J Physiol Cell Physiol. 2014. PMID: 25080489 Free PMC article.
-
Growth hormone secretagogues prevent dysregulation of skeletal muscle calcium homeostasis in a rat model of cisplatin-induced cachexia.J Cachexia Sarcopenia Muscle. 2017 Jun;8(3):386-404. doi: 10.1002/jcsm.12185. Epub 2017 Mar 10. J Cachexia Sarcopenia Muscle. 2017. PMID: 28294567 Free PMC article.
-
ClC-1 chloride channels: state-of-the-art research and future challenges.Front Cell Neurosci. 2015 Apr 27;9:156. doi: 10.3389/fncel.2015.00156. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25964741 Free PMC article. Review.
References
-
- Bodine SC, Latres E, Baumhueter S, Lai VK-M, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan Z-Q, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. - PubMed
-
- Bryant SH, Conte-Camerino D. Chloride channel regulation in the skeletal muscle of normal and myotonic goats. Pflugers Arch. 1991;417:605–610. - PubMed
-
- Chen MF, Jockusch H. Role of phosphorylation and physiological state in the regulation of the muscular chloride channel ClC-1: a voltage-clamp study on isolated M. interosseus fibers. Biochem Biophys Res Commun. 1999;261:528–533. - PubMed
-
- Choi I, Lee K, Kim M, Lee M, Park K. Differential activation of stress-responsive signaling proteins associated with altered loading in a rat skeletal muscle. J Cell Biochem. 2005;96:1231–1243. - PubMed
-
- Cleland PJ, Appleby GJ, Rattigan S, Clark MG. Exercise-induced translocation of protein kinase C and production of diacylglycerol and phosphatidic acid in rat skeletal muscle in vivo. Relationship to changes in glucose transport. J Biol Chem. 1989;264:17704–17711. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

