Neurexin Ibeta and neuroligin are localized on opposite membranes in mature central synapses
- PMID: 17868325
- PMCID: PMC2517655
- DOI: 10.1111/j.1471-4159.2007.04918.x
Neurexin Ibeta and neuroligin are localized on opposite membranes in mature central synapses
Abstract
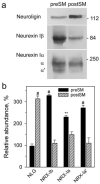
Synaptogenesis requires formation of trans-synaptic complexes between neuronal cell-adhesion receptors. Heterophilic receptor pairs, such as neurexin Ibeta and neuroligin, can mediate distinct intracellular signals and form different cytoplasmic scaffolds in the pre- and post-synaptic neuron, and may be particularly important for synaptogenesis. However, the functions of neurexin and neuroligin depend on their distribution in the synapse. Neuroligin has been experimentally assigned to the post-synaptic membrane, while the localization of neurexin remains unclear. To study the subcellular distribution of neurexin Ibeta and neuroligin in mature cerebrocortical synapses, we have developed a novel method for the physical separation of junctional membranes and their direct analysis by western blotting. Using urea and dithiothreitol, we disrupted trans-synaptic protein links, without dissolving the lipid phase, and fractionated the pre- and post-synaptic membranes. The purity of these fractions was validated by electron microscopy and western blotting using multiple synaptic markers. A quantitative analysis has confirmed that neuroligin is localized strictly in the post-synaptic membrane. We have also demonstrated that neurexin Ibeta is largely (96%) pre-synaptic. Thus, neurexin Ibeta and neuroligin normally form trans-synaptic complexes and can transduce bidirectional signals.
Figures



Similar articles
-
Imaging activity-dependent regulation of neurexin-neuroligin interactions using trans-synaptic enzymatic biotinylation.Cell. 2010 Oct 29;143(3):456-69. doi: 10.1016/j.cell.2010.09.025. Epub 2010 Oct 7. Cell. 2010. Retraction in: Cell. 2013 Feb 14;152(4):923. doi: 10.1016/j.cell.2013.01.021. PMID: 20933261 Free PMC article. Retracted.
-
The postsynaptic adenomatous polyposis coli (APC) multiprotein complex is required for localizing neuroligin and neurexin to neuronal nicotinic synapses in vivo.J Neurosci. 2010 Aug 18;30(33):11073-85. doi: 10.1523/JNEUROSCI.0983-10.2010. J Neurosci. 2010. PMID: 20720115 Free PMC article.
-
A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins.Neuron. 2005 Oct 20;48(2):229-36. doi: 10.1016/j.neuron.2005.08.026. Neuron. 2005. PMID: 16242404
-
Neurexin-neuroligin signaling in synapse development.Curr Opin Neurobiol. 2007 Feb;17(1):43-52. doi: 10.1016/j.conb.2007.01.011. Epub 2007 Feb 1. Curr Opin Neurobiol. 2007. PMID: 17275284 Free PMC article. Review.
-
Synaptic adhesion molecules.Curr Opin Cell Biol. 2003 Oct;15(5):621-32. doi: 10.1016/s0955-0674(03)00107-8. Curr Opin Cell Biol. 2003. PMID: 14519398 Review.
Cited by
-
Bridging the synaptic gap: neuroligins and neurexin I in Apis mellifera.PLoS One. 2008;3(10):e3542. doi: 10.1371/journal.pone.0003542. Epub 2008 Oct 31. PLoS One. 2008. PMID: 18974885 Free PMC article.
-
Penelope's web: using alpha-latrotoxin to untangle the mysteries of exocytosis.J Neurochem. 2009 Oct;111(2):275-90. doi: 10.1111/j.1471-4159.2009.06329.x. Epub 2009 Aug 13. J Neurochem. 2009. PMID: 19682210 Free PMC article. Review.
-
Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation.EMBO J. 2009 Oct 21;28(20):3244-55. doi: 10.1038/emboj.2009.249. Epub 2009 Sep 3. EMBO J. 2009. PMID: 19730411 Free PMC article.
-
Neuroligins and neurexins link synaptic function to cognitive disease.Nature. 2008 Oct 16;455(7215):903-11. doi: 10.1038/nature07456. Nature. 2008. PMID: 18923512 Free PMC article. Review.
-
Differential expression of presynaptic genes in a rat model of postnatal hypoxia: relevance to schizophrenia.Eur Arch Psychiatry Clin Neurosci. 2010 Nov;260 Suppl 2(Suppl 2):S81-9. doi: 10.1007/s00406-010-0159-1. Epub 2010 Oct 14. Eur Arch Psychiatry Clin Neurosci. 2010. PMID: 20945070 Free PMC article.
References
-
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. - PubMed
-
- Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. - PubMed
-
- Chubykin AA, Liu X, Comoletti D, Tsigelny I, Taylor P, Sudhof TC. Dissection of synapse induction by neuroligins: effect of a neuroligin mutation associated with autism. J. Biol. Chem. 2005;280:22365–22374. - PubMed
-
- Chung YH, Shin C, Kim MJ, Lee BK, Cha CI. Immunohistochemical study on the distribution of six members of the Kv1 channel subunits in the rat cerebellum. Brain Res. 2001;895:173–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

