Cable properties and propagation velocity in a long single chain of simulated myocardial cells
- PMID: 17868460
- PMCID: PMC2071913
- DOI: 10.1186/1742-4682-4-36
Cable properties and propagation velocity in a long single chain of simulated myocardial cells
Abstract
Background: Propagation of simulated action potentials (APs) was previously studied in short single chains and in two-dimensional sheets of myocardial cells 123. The present study was undertaken to examine propagation in a long single chain of cells of various lengths, and with varying numbers of gap-junction (g-j) channels, and to compare propagation velocity with the cable properties such as the length constant (lambda).
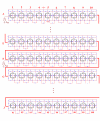
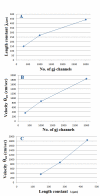
Methods and results: Simulations were carried out using the PSpice program as previously described. When the electric field (EF) mechanism was dominant (0, 1, and 10 gj-channels), the longer the chain length, the faster the overall velocity (theta(ov)). There seems to be no simple explanation for this phenomenon. In contrast, when the local-circuit current mechanism was dominant (100 gj-channels or more), theta(ov) was slightly slowed with lengthening of the chain. Increasing the number of gj-channels produced an increase in theta(ov) and caused the firing order to become more uniform. The end-effect was more pronounced at longer chain lengths and at greater number of gj-channels. When there were no or only few gj-channels (namely, 0, 10, or 30), the voltage change (DeltaV(m)) in the two contiguous cells (#50 & #52) to the cell injected with current (#51) was nearly zero, i.e., there was a sharp discontinuity in voltage between the adjacent cells. When there were many gj-channels (e.g., 300, 1000, 3000), there was an exponential decay of voltage on either side of the injected cell, with the length constant (lambda) increasing at higher numbers of gj-channels. The effect of increasing the number of gj-channels on increasing lambda was relatively small compared to the larger effect on theta(ov). theta(ov) became very non-physiological at 300 gj-channels or higher.
Conclusion: Thus, when there were only 0, 1, or 10 gj-channels, theta(ov) increased with increase in chain length, whereas at 100 gj-channels or higher, theta(ov) did not increase with chain length. When there were only 0, 10, or 30 gj-channels, there was a very sharp decrease in DeltaV(m) in the two contiguous cells on either side of the injected cell, whereas at 300, 1000, or 3000 gj-channels, the voltage decay was exponential along the length of the chain. The effect of increasing the number of gj-channels on spread of current was relatively small compared to the large effect on theta(ov).
Figures







References
-
- Sperelakis N, Ramasamy L. Propagation in cardiac muscle and smooth muscle based on electric field transmission at cell junctions: An analysis by PSpice. IEEE-EMB. 2002;21:130. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

