Calcium-sensitive regulation of monoamine oxidase-A contributes to the production of peroxyradicals in hippocampal cultures: implications for Alzheimer disease-related pathology
- PMID: 17868476
- PMCID: PMC2048967
- DOI: 10.1186/1471-2202-8-73
Calcium-sensitive regulation of monoamine oxidase-A contributes to the production of peroxyradicals in hippocampal cultures: implications for Alzheimer disease-related pathology
Abstract
Background: Calcium (Ca2+) has recently been shown to selectively increase the activity of monoamine oxidase-A (MAO-A), a mitochondria-bound enzyme that generates peroxyradicals as a natural by-product of the deamination of neurotransmitters such as serotonin. It has also been suggested that increased intracellular free Ca2+ levels as well as MAO-A may be contributing to the oxidative stress associated with Alzheimer disease (AD).
Results: Incubation with Ca2+ selectively increases MAO-A enzymatic activity in protein extracts from mouse hippocampal HT-22 cell cultures. Treatment of HT-22 cultures with the Ca2+ ionophore A23187 also increases MAO-A activity, whereas overexpression of calbindin-D28K (CB-28K), a Ca2+-binding protein in brain that is greatly reduced in AD, decreases MAO-A activity. The effects of A23187 and CB-28K are both independent of any change in MAO-A protein or gene expression. The toxicity (via production of peroxyradicals and/or chromatin condensation) associated with either A23187 or the AD-related beta-amyloid peptide, which also increases free intracellular Ca2+, is attenuated by MAO-A inhibition in HT-22 cells as well as in primary hippocampal cultures.
Conclusion: These data suggest that increases in intracellular Ca2+ availability could contribute to a MAO-A-mediated mechanism with a role in AD-related oxidative stress.
Figures
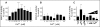

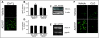


References
-
- Magyar K, Palfi M, Tabi T, Kalasz H, Szende B, Szoko E. Pharmacological aspects of (-)-deprenyl. Current medicinal chemistry. 2004;11:2017–2031. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

