Structural and biochemical basis for polyamine binding to the Tp0655 lipoprotein of Treponema pallidum: putative role for Tp0655 (TpPotD) as a polyamine receptor
- PMID: 17868688
- PMCID: PMC2094014
- DOI: 10.1016/j.jmb.2007.08.018
Structural and biochemical basis for polyamine binding to the Tp0655 lipoprotein of Treponema pallidum: putative role for Tp0655 (TpPotD) as a polyamine receptor
Abstract
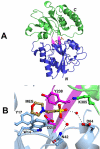
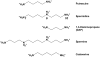
Tp0655 of Treponema pallidum, the causative agent of syphilis, is predicted to be a 40 kDa membrane lipoprotein. Previous sequence analysis of Tp0655 noted its homology to polyamine-binding proteins of the bacterial PotD family, which serve as periplasmic ligand-binding proteins of ATP-binding-cassette (ABC) transport systems. Here, the 1.8 A crystal structure of Tp0655 demonstrated structural homology to Escherichia coli PotD and PotF. The latter two proteins preferentially bind spermidine and putrescine, respectively. All of these proteins contain two domains that sandwich the ligand between them. The ligand-binding site of Tp0655 can be occupied by 2-(N-morpholino)ethanesulfanoic acid, a component of the crystallization medium. To discern the polyamine binding preferences of Tp0655, the protein was subjected to isothermal titration calorimetric experiments. The titrations established that Tp0655 binds polyamines avidly, with a marked preference for putrescine (Kd=10 nM) over spermidine (Kd=430 nM), but the related compounds cadaverine and spermine did not bind. Structural comparisons and structure-based sequence analyses provide insights into how polyamine-binding proteins recognize their ligands. In particular, these comparisons allow the derivation of rules that may be used to predict the function of other members of the PotD family. The sequential, structural, and functional homology of Tp0655 to PotD and PotF prompt the conclusion that the former likely is the polyamine-binding component of an ABC-type polyamine transport system in T. pallidum. We thus rename Tp0655 as TpPotD. The ramifications of TpPotD as a polyamine-binding protein to the parasitic strategy of T. pallidum are discussed.
Figures

Similar articles
-
Structural basis of substrate binding specificity revealed by the crystal structures of polyamine receptors SpuD and SpuE from Pseudomonas aeruginosa.J Mol Biol. 2012 Mar 9;416(5):697-712. doi: 10.1016/j.jmb.2012.01.010. Epub 2012 Jan 28. J Mol Biol. 2012. PMID: 22300763
-
Crystal structure of PotD, the primary receptor of the polyamine transport system in Escherichia coli.J Biol Chem. 1996 Apr 19;271(16):9519-25. doi: 10.1074/jbc.271.16.9519. J Biol Chem. 1996. PMID: 8621624
-
Change in protein-ligand specificity through binding pocket grafting.J Struct Biol. 2014 Feb;185(2):186-92. doi: 10.1016/j.jsb.2013.06.002. Epub 2013 Jun 17. J Struct Biol. 2014. PMID: 23792166
-
Polyamine transport in bacteria and yeast.Biochem J. 1999 Dec 15;344 Pt 3(Pt 3):633-42. Biochem J. 1999. PMID: 10585849 Free PMC article. Review.
-
Polyamine uptake systems in Escherichia coli.Res Microbiol. 2001 Apr-May;152(3-4):271-8. doi: 10.1016/s0923-2508(01)01198-6. Res Microbiol. 2001. PMID: 11421274 Review.
Cited by
-
High-precision, automated integration of multiple isothermal titration calorimetric thermograms: new features of NITPIC.Methods. 2015 Apr;76:87-98. doi: 10.1016/j.ymeth.2014.11.024. Epub 2014 Dec 15. Methods. 2015. PMID: 25524420 Free PMC article.
-
Functional clues from the crystal structure of an orphan periplasmic ligand-binding protein from Treponema pallidum.Protein Sci. 2017 Apr;26(4):847-856. doi: 10.1002/pro.3133. Epub 2017 Mar 23. Protein Sci. 2017. PMID: 28168761 Free PMC article.
-
Surface immunolabeling and consensus computational framework to identify candidate rare outer membrane proteins of Treponema pallidum.Infect Immun. 2010 Dec;78(12):5178-94. doi: 10.1128/IAI.00834-10. Epub 2010 Sep 27. Infect Immun. 2010. PMID: 20876295 Free PMC article.
-
Purification, crystallization and preliminary X-ray analysis of TP0435 (Tp17) from the syphilis spirochete Treponema pallidum.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013 Apr 1;69(Pt 4):453-5. doi: 10.1107/S1744309113006246. Epub 2013 Mar 28. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013. PMID: 23545658 Free PMC article.
-
The Treponema pallidum Outer Membrane.Curr Top Microbiol Immunol. 2018;415:1-38. doi: 10.1007/82_2017_44. Curr Top Microbiol Immunol. 2018. PMID: 28849315 Free PMC article. Review.
References
-
- Chao JR, Khurana RN, Fawzi AA, Reddy HS, Rao NA. Syphilis: reemergence of an old adversary. Ophthamol. 2006;113:2074–2079. - PubMed
-
- Kerani RP, Handsfield HH, Stenger MS, Shafii T, Zick E, et al. Rising rates of syphilis in the era of syphilis elimination. Sex. Transm. Dis. 2007;34:154–161. - PubMed
-
- Radolf JD. Role of outer membrane architecture in immune evasion by Treponema pallidum and Borrelia burgdorferi. Trends Microbiol. 1994;2:307–311. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

