Intravascular drug release kinetics dictate arterial drug deposition, retention, and distribution
- PMID: 17868948
- PMCID: PMC2702153
- DOI: 10.1016/j.jconrel.2007.06.025
Intravascular drug release kinetics dictate arterial drug deposition, retention, and distribution
Abstract
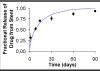
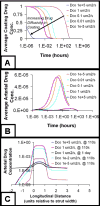
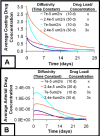
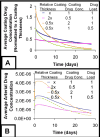
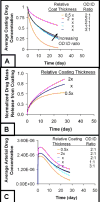
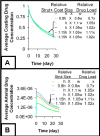
Millions of patients worldwide have received drug-eluting stents to reduce their risk for in-stent restenosis. The efficacy and toxicity of these local therapeutics depend upon arterial drug deposition, distribution, and retention. To examine how administered dose and drug release kinetics control arterial drug uptake, a model was created using principles of computational fluid dynamics and transient drug diffusion-convection. The modeling predictions for drug elution were validated using empiric data from stented porcine coronary arteries. Inefficient, minimal arterial drug deposition was predicted when a bolus of drug was released and depleted within seconds. Month-long stent-based drug release efficiently delivered nearly continuous drug levels, but the slow rate of drug presentation limited arterial drug uptake. Uptake was only maximized when the rates of drug release and absorption matched, which occurred for hour-long drug release. Of the two possible means for increasing the amount of drug on the stent, modulation of drug concentration potently impacts the magnitude of arterial drug deposition, while changes in coating drug mass affect duration of release. We demonstrate the importance of drug release kinetics and administered drug dose in governing arterial drug uptake and suggest novel drug delivery strategies for controlling spatio-temporal arterial drug distribution.
Figures


References
-
- Axel DI, Kunert W, Goggelmann C, Oberhoff M, Herdeg C, Kuttner A, Wild DH, Brehm BR, Riessen R, Koveker G, Karsch KR. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96(2):636–645. - PubMed
-
- Hwang CW, Wu D, Edelman ER. Physiological transport forces govern drug distribution for stent-based delivery. Circulation. 2001;104(5):600–605. - PubMed
-
- Creel CJ, Lovich MA, Edelman ER. Arterial paclitaxel distribution and deposition. Circ Res. 2000;86(8):879–884. - PubMed
-
- Hausleiter J, Kastrati A, Mehilli J, Vogeser M, Zohlnhofer D, Schuhlen H, Goos C, Pache J, Dotzer F, Pogatsa-Murray G, Dirschinger J, Heemann U, Schomig A. Randomized, double-blind, placebo-controlled trial of oral sirolimus for restenosis prevention in patients with in-stent restenosis: the Oral Sirolimus to Inhibit Recurrent Instent Stenosis (OSIRIS) trial. Circulation. 2004;110(7):790–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

