Modulation of endothelial cell thrombomodulin by PPAR ligands--variation according to environment
- PMID: 17869327
- PMCID: PMC2577783
- DOI: 10.1016/j.thromres.2007.08.007
Modulation of endothelial cell thrombomodulin by PPAR ligands--variation according to environment
Abstract
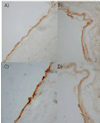
Introduction: Thrombomodulin (TM) is an important anti-coagulant protein that is down-regulated on endothelial cells overlying atherosclerotic plaques. We investigated the effects of the peroxisome proliferator-activated receptor (PPAR) ligands, fenofibrate and rosiglitazone, on the expression of TM ex vivo by advanced carotid atheromas, and in vitro by endothelial cells.
Methods: Adjacent carotid atheroma biopsies were incubated in vehicle control or PPAR ligand in explant culture for 4 days. Human aortic endothelial cells were incubated with PPAR ligands in vitro. TM expression was measured by Western blotting and flow cytometry. TM activity was assessed by generation of activated protein C.
Results: The PPAR-alpha activator, fenofibrate, up-regulated total TM expression within carotid explants by 1.7-fold (P<0.001) with no effect on activity. Rosiglitazone treatment had no effect on protein levels but reduced activity by 73% of the control (P<0.05). We noted disparate effects of PPAR ligands in atheroma samples from different patients and postulated that the response of endothelial cells to medication was influenced by the atheromatous environment. Incubation of human aortic endothelial cells with fenofibrate alone led to a dose-dependent increase in TM expression (P<0.05), however, in the presence of oxidized LDL a dose-dependent reduction in TM expression was induced by fenofibrate (P<0.05).
Conclusions: The ability of fenofibrate to increase endothelial cell and carotid atheroma TM protein expression suggests a potential therapeutic role for this medication. The response to PPAR ligands likely varies depending on the exact constituents of individual atherosclerotic plaques, such as the relative amount of oxidized LDL.
Figures







References
-
- Weiler H, Isermann BH. Thrombomodulin. J Thromb Haemost. 2003;1:1515–1524. - PubMed
-
- Seigneur M, Dufourcq P, Conri C, Constans J, Mercie P, Pruvost A, Amiral J, Midy D, Baste J, Boisseau MR. Levels of plasma thrombomodulin are increased in atheromatous arterial disease. Thromb Res. 1993;71:423–431. - PubMed
-
- Gerdes VEA, Kremer Hovinga JA, ten Cate H, Brandjes DPM, Buller HR. Soluble thrombomodulin in patients with established atherosclerosis. J Thromb Haemost. 2004;2:200–201. - PubMed
-
- Wu KK, Aleksic N, Ballantyne CM, Ahn C, Juneja H, Boerwinkle E. Interaction between soluble thrombomodulin and intercellular adhesion molecule-1 in predicting risk of coronary heart disease. Circulation. 2003;107:1729–1732. - PubMed
-
- Suzuki K, Stenflo J, Dahlback B, Teodorsson B. Inactivationof human coagulation factor V by activated protein C. J Biol Chem. 1983;258:1914–1920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

