Transport of beads by several kinesin motors
- PMID: 17872957
- PMCID: PMC2157241
- DOI: 10.1529/biophysj.106.097881
Transport of beads by several kinesin motors
Abstract
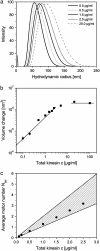
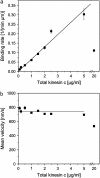
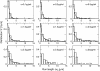
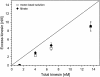
The movements of beads pulled by several kinesin-1 (conventional kinesin) motors are studied both theoretically and experimentally. While the velocity is approximately independent of the number of motors pulling the beads, the walking distance or run-length is strongly increased when more motors are involved. Run-length distributions are measured for a wide range of motor concentrations and matched to theoretically calculated distributions using only two global fit parameters. In this way, the maximal number of motors pulling the beads is estimated to vary between two and seven motors for total kinesin concentrations between 0.1 and 2.5 microg/ml or between 0.27 and 6.7 nM. In the same concentration regime, the average number of pulling motors is found to lie between 1.1 and 3.2 motors.
Figures
References
-
- Howard, J. 2001. Mechanics of Motor Proteins and the Cytoskeleton. Sinauer Associates, Sunderland, MA.
-
- Schliwa, M. (Editor.). 2003. Molecular Motors. Wiley-VCH, Weinheim, Germany.
-
- Howard, J., A. J. Hudspeth, and R. D. Vale. 1989. Movement of microtubules by single kinesin molecules. Nature. 342:154–158. - PubMed
-
- Block, S. M., L. S. B. Goldstein, and B. J. Schnapp. 1990. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 348:348–352. - PubMed
-
- Visscher, K., M. J. Schnitzer, and S. M. Block. 1999. Single kinesin molecules studied with a molecular force clamp. Nature. 400:184–189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

