Resveratrol (trans-3,5,4'-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor
- PMID: 17872969
- PMCID: PMC4796949
- DOI: 10.1124/mol.107.038984
Resveratrol (trans-3,5,4'-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor
Abstract
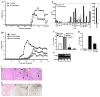
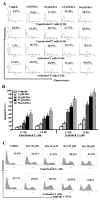
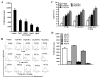
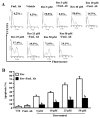
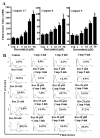
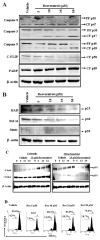
Resveratrol (trans-3,5,4'-trihydroxystilbene), a polyphenolic compound found in plant products, including red grapes, exhibits anticancer, antioxidant, and anti-inflammatory properties. Using an animal model of multiple sclerosis (MS), we investigated the use of resveratrol for the treatment of autoimmune diseases. We observed that resveratrol treatment decreased the clinical symptoms and inflammatory responses in experimental allergic encephalomyelitis (EAE)-induced mice. Furthermore, we observed significant apoptosis in inflammatory cells in spinal cord of EAE-induced mice treated with resveratrol compared with the control mice. Resveratrol administration also led to significant down-regulation of certain cytokines and chemokines in EAE-induced mice including tumor necrosis factor-alpha, interferon-gamma, interleukin (IL)-2, IL-9, IL-12, IL-17, macrophage inflammatory protein-1alpha (MIP-1alpha), monocyte chemoattractant protein-1 (MCP-1), regulated on activation normal T-cell expressed and secreted (RANTES), and Eotaxin. In vitro studies on the mechanism of action revealed that resveratrol triggered high levels of apoptosis in activated T cells and to a lesser extent in unactivated T cells. Moreover, resveratrol-induced apoptosis was mediated through activation of aryl hydrocarbon receptor (AhR) and estrogen receptor (ER) and correlated with up-regulation of AhR, Fas, and FasL expression. In addition, resveratrol-induced apoptosis in primary T cells correlated with cleavage of caspase-8, caspase-9, caspase-3, poly(ADP-ribose) polymerase, and release of cytochrome c. Data from the present study demonstrate, for the first time, the ability of resveratrol to trigger apoptosis in activated T cells and its potential use in the treatment of inflammatory and autoimmune diseases including, MS.
Figures



References
-
- Atten MJ, Godoy-Romero E, Attar BM, Milson T, Zopel M, Holian O. Resveratrol regulates cellular PKC alpha and delta to inhibit growth and induce apoptosis in gastric cancer cells. Invest New Drugs. 2005;23:111–119. - PubMed
-
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. - PubMed
-
- Bernhard D, Tinhofer I, Tonko M, Hubl H, Ausserlechner MJ, Greil R, Kofler R, Csordas A. Resveratrol causes arrest in the S-phase prior to Fas-independent apoptosis in CEM-C7H2 acute leukemia cells. Cell Death Differ. 2000;7:834–842. - PubMed
-
- Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–7463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL058641/HL/NHLBI NIH HHS/United States
- R01-AI053703/AI/NIAID NIH HHS/United States
- R01 ES009098/ES/NIEHS NIH HHS/United States
- P01-AT003961/AT/NCCIH NIH HHS/United States
- P01 AT003961/AT/NCCIH NIH HHS/United States
- R01 HL058641/HL/NHLBI NIH HHS/United States
- F31 ES011562/ES/NIEHS NIH HHS/United States
- R01 DA016545/DA/NIDA NIH HHS/United States
- R21-DA014885/DA/NIDA NIH HHS/United States
- R01-ES09098/ES/NIEHS NIH HHS/United States
- R01 AI053703/AI/NIAID NIH HHS/United States
- R01 AI058300/AI/NIAID NIH HHS/United States
- F31-ES11562/ES/NIEHS NIH HHS/United States
- R01-DA016545/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

