Myosin II co-chaperone general cell UNC-45 overexpression is associated with ovarian cancer, rapid proliferation, and motility
- PMID: 17872978
- PMCID: PMC2043524
- DOI: 10.2353/ajpath.2007.070325
Myosin II co-chaperone general cell UNC-45 overexpression is associated with ovarian cancer, rapid proliferation, and motility
Abstract
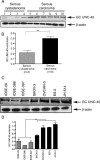
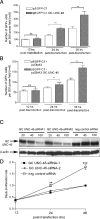
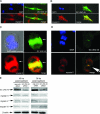
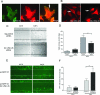
Both tumor cell proliferation and metastasis are dependent on myosin II. Because UNC-45 is required to chaperone the assembly of a functional myosin II motor, we examined the expression of the general cell (GC) UNC-45 isoform in ovarian tumors. Serous carcinoma expressed elevated levels of GC UNC-45 compared with normal ovarian surface epithelium and benign cystadenoma. High-stage exhibited greater GC UNC-45 expression than low-stage serous carcinoma. Similarly, GC UNC-45 transcripts and protein levels were higher in ovarian cell lines than in immortalized ovarian surface epithelial cells. Elevation of GC UNC-45 levels by ectopic expression enhanced the rate of ovarian cancer cell proliferation, whereas siRNA knockdown of GC UNC-45 suppressed proliferation without altering myosin II levels. GC UNC-45 and myosin II were diffuse within the cytoplasm of confluent interphase cells, but both accumulated together at the cleavage furrow during cytokinesis. GC UNC-45 and myosin II also trafficked to the leading edges of ovarian cancer cells induced to move in a scratch assay. Knockdown of GC UNC-45 reduced the spreading ability of ovarian cancer cells whereas it was enhanced by GC UNC-45 overexpression. In sum, these findings implicate elevated GC UNC-45 protein expression in ovarian carcinoma proliferation and metastasis.
Figures


References
-
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. - PubMed
-
- Bazzaro M, Lee MK, Zoso A, Stirling WL, Santillan A, Shih Ie M, Roden RB. Ubiquitin-proteasome system stress sensitizes ovarian cancer to proteasome inhibitor-induced apoptosis. Cancer Res. 2006;66:3754–3763. - PubMed
-
- Balasubramanian MK, Bi E, Glotzer M. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr Biol. 2004;14:R806–R818. - PubMed
-
- Bresnick AR. Molecular mechanisms of nonmuscle myosin-II regulation. Curr Opin Cell Biol. 1999;11:26–33. - PubMed
-
- Edwards KA, Kiehart DP. Drosophila nonmuscle myosin II has multiple essential roles in imaginal disc and egg chamber morphogenesis. Development. 1996;122:1499–1511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

