Thioredoxin reductase is essential for thiol/disulfide redox control and oxidative stress survival of the anaerobe Bacteroides fragilis
- PMID: 17873045
- PMCID: PMC2168685
- DOI: 10.1128/JB.00714-07
Thioredoxin reductase is essential for thiol/disulfide redox control and oxidative stress survival of the anaerobe Bacteroides fragilis
Abstract
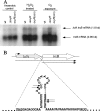
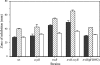
Results of this study showed that the anaerobic, opportunistic pathogen Bacteroides fragilis lacks the glutathione/glutaredoxin redox system and possesses an extensive number of putative thioredoxin (Trx) orthologs. Analysis of the genome sequence revealed six Trx orthologs and an absence of genes required for synthesis of glutathione and glutaredoxins. In addition, it was shown that the thioredoxin reductase (TrxB)/Trx system is the major or sole redox system for thiol/disulfide cellular homeostasis in this anaerobic bacterium. Expression of the B. fragilis trxB gene was induced following treatment with diamide or H(2)O(2) or exposure to oxygen. This inducible trxB expression was OxyR independent. Northern blot hybridization analysis showed that the trxB mRNA was cotranscribed with lolA as a bicistronic transcript or was present as a monocistronic transcript that was also highly induced under the same conditions. The role of LolA, a prokaryotic periplasmic lipoprotein-specific molecular chaperone in the thiol/disulfide redox system, is unknown. A trxB deletion mutant was more sensitive to the effects of diamide and oxygen than the parent strain. In addition, the trxB mutant was unable to grow in culture media without addition of a reductant. Furthermore, the trxB mutant was not able to induce intraabdominal abscess formation in a mouse model, whereas the parent strain was. Taken together, these data strongly suggest that TrxB/Trx is the major, if not the sole, thiol/disulfide redox system in this anaerobe required for survival and abscess formation in a peritoneal cavity infection model.
Figures





References
-
- Andreesen, J. R., M. Wagner, D. Sonntag, M. Kohlstock, C. Harms, T. Gursinsky, J. Jager, T. Parther, U. Kabisch, A. Grantzdorffer, A. Pich, and B. Sohling. 1999. Various functions of selenols and thiols in anaerobic gram-positive, amino acids-utilizing bacteria. Biofactors 10:263-270. - PubMed
-
- Arner, E. S., and A. Holmgren. 2000. Physiological functions of thioredoxins and thioredoxin reductases. Eur. J. Biochem. 267:6102-6109. - PubMed
-
- Baughn, A. D., and M. H. Malamy. 2004. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 427:441-444. - PubMed
-
- Bayley, D. P., E. R. Rocha, and C. J. Smith. 2000. Analysis of cepA and other Bacteroides fragilis genes reveals a unique promoter structure. FEMS Microbiol. Lett. 193:149-154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

