Heterologous production of dihomo-gamma-linolenic acid in Saccharomyces cerevisiae
- PMID: 17873077
- PMCID: PMC2074983
- DOI: 10.1128/AEM.01008-07
Heterologous production of dihomo-gamma-linolenic acid in Saccharomyces cerevisiae
Abstract
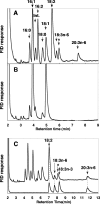
To make dihomo-gamma-linolenic acid (DGLA) (20:3n-6) in Saccharomyces cerevisiae, we introduced Kluyveromyces lactis Delta12 fatty acid desaturase, rat Delta6 fatty acid desaturase, and rat elongase genes. Because Fad2p is able to convert the endogenous oleic acid to linoleic acid, this allowed DGLA biosynthesis without the need to supply exogenous fatty acids on the media. Medium composition, cultivation temperature, and incubation time were examined to improve the yield of DGLA. Fatty acid content was increased by changing the medium from a standard synthetic dropout medium to a nitrogen-limited minimal medium (NSD). Production of DGLA was higher in the cells grown at 15 degrees C than in those grown at 20 degrees C, and no DGLA production was observed in the cells grown at 30 degrees C. In NSD at 15 degrees C, fatty acid content increased up until day 7 and decreased after day 10. When the cells were grown in NSD for 7 days at 15 degrees C, the yield of DGLA reached 2.19 microg/mg of cells (dry weight) and the composition of DGLA to total fatty acids was 2.74%. To our knowledge, this is the first report describing the production of polyunsaturated fatty acids in S. cerevisiae without supplying the exogenous fatty acids.
Figures



References
-
- Aki, T., Y. Shimada, K. Inagaki, H. Higashimoto, S. Kawamoto, S. Shigeta, K. Ono, and O. Suzuki. 1999. Molecular cloning and functional characterization of rat delta-6 fatty acid desaturase. Biochem. Biophys. Res. Commun. 255:575-579. - PubMed
-
- Broun, P., S. Gettner, and C. Somerville. 1999. Genetic engineering of plant lipids. Annu. Rev. Nutr. 19:197-216. - PubMed
-
- Calvo, A. M., H. W. Gardner, and N. P. Keller. 2001. Genetic connection between fatty acid metabolism and sporulation in Aspergillus nidulans. J. Biol. Chem. 276:25766-25774. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

