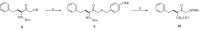Synthesis of sulfonamides and evaluation of their histone deacetylase (HDAC) activity
- PMID: 17873846
- PMCID: PMC6149482
- DOI: 10.3390/12051125
Synthesis of sulfonamides and evaluation of their histone deacetylase (HDAC) activity
Erratum in
- Molecules. 2009;14(5):1950-1. Avery, Mitchell A [added]
Abstract
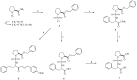
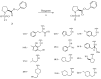
A simple synthesis of sulfonamides 4-22 as novel histone deacetylase (HDAC) inhibitors is described. The key synthetic strategies involve N-sulfonylation of L-proline benzyl ester hydrochloride (2) and coupling reaction of N-sulfonyl chloride 3 with amines in high yields. It was found that several compounds showed good cellular potency with the most potent compound 20 exhibiting an IC50 = 2.8 microM in vitro.
Figures
Similar articles
-
Design, synthesis and evaluation of belinostat analogs as histone deacetylase inhibitors.Future Med Chem. 2019 Nov;11(21):2765-2778. doi: 10.4155/fmc-2018-0587. Epub 2019 Nov 8. Future Med Chem. 2019. PMID: 31702394 Free PMC article.
-
Development of potential antitumor agents. Synthesis and biological evaluation of a new set of sulfonamide derivatives as histone deacetylase inhibitors.J Med Chem. 2003 Feb 27;46(5):820-30. doi: 10.1021/jm020377a. J Med Chem. 2003. PMID: 12593661
-
Synthesis and evaluation of lysine derived sulfamides as histone deacetylase inhibitors.Bioorg Med Chem Lett. 2009 Apr 1;19(7):1866-70. doi: 10.1016/j.bmcl.2009.02.075. Epub 2009 Feb 23. Bioorg Med Chem Lett. 2009. PMID: 19272776
-
Discovery of pyrimidyl-5-hydroxamic acids as new potent histone deacetylase inhibitors.Eur J Med Chem. 2005 Jun;40(6):597-606. doi: 10.1016/j.ejmech.2005.01.008. Epub 2005 Mar 3. Eur J Med Chem. 2005. PMID: 15922843
-
Histone deacetylase inhibitors from the rhizomes of Zingiber zerumbet.Pharmazie. 2008 Oct;63(10):774-6. Pharmazie. 2008. PMID: 18972844
Cited by
-
Novel Derivatives of 3-Amino-4-hydroxy-benzenesulfonamide: Synthesis, Binding to Carbonic Anhydrases, and Activity in Cancer Cell 2D and 3D Cultures.Int J Mol Sci. 2025 Jul 4;26(13):6466. doi: 10.3390/ijms26136466. Int J Mol Sci. 2025. PMID: 40650242 Free PMC article.
-
Recent advances in anticancer mechanisms of molecular glue degraders: focus on RBM39-dgrading synthetic sulfonamide such as indisulam, E7820, tasisulam, and chloroquinoxaline sulfonamide.Genes Genomics. 2024 Dec;46(12):1345-1361. doi: 10.1007/s13258-024-01565-z. Epub 2024 Sep 13. Genes Genomics. 2024. PMID: 39271535 Review.
-
Synthesis of brominated 2-phenitidine derivatives as valuable inhibitors of cholinesterases for the treatment of Alzheimer's disease.Iran J Pharm Res. 2014 Winter;13(1):87-94. Iran J Pharm Res. 2014. PMID: 24734059 Free PMC article.
References
-
-
Vigushin D. M., Coombes R. C. Histone deacetylase inhibitors in cancer treatment. Anti–Cancer Drugs. 2002;13:1–13. Vigushin D. M., Coombes R. C. Targeted histone deacetylase inhibition for cancer therapy. Curr. Cancer Drug Targ. 2004;4:205–218.
-
-
-
Mai A., Massa S., Rotili D., Cerbara I., Valente S., Pezzi R., Simeoni S., Ragno R. Histone deacetylation in epigenetics: an attractive target for anticancer therapy. Med. Res. Rev. 2005;25:261–309. Dangond F., Henriksson M., Zardo G., Caiafa P., Ekstrom T. J., Gray S. G. Differential expression of class I HDACs: roles of cell density and cell cycle. Int. J. Oncol. 2001;19:773–777.
-
-
-
Imre G., Gekeler V., Leja A., Beckers T., Boehm M. Histone deacetylase inhibitors suppress the inducibility of nuclear factor-kappaB by tumor necrosis factor-alpha receptor-1 down-regulation. Cancer Res. 2006;66:5409–5418. O'Connor O. A., Heaney M. L., Schwartz L., Richardson S., Willim R., MacGregor–Cortelli B., Curly T., Moskowitz C., Portlock C., Horwitz S., Zelenetz A. D., Frankel S., Richon V., Marks P., Kelly W. K. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J. Clin. Oncol. 2006;24:166–173. Mayo M. W., Denlinger C. E., Broad R. M., Yeung F., Reilly E. T., Shi Y., Jones D. R. Ineffectiveness of histone deacetylase inhibitors to induce apoptosis involves the transcriptional activation of NF-kappa B through the Akt pathway. J. Biol. Chem. 2003;278:18980–18989.
-
-
-
Suzuki T., Nagano Y., Kouketsu A., Matsuura A., Maruyama S., Kurotaki M., Nakagawa H., Miyata N. Novel inhibitors of human histone deacetylases: design, synthesis, enzyme inhibition, and cancer cell growth inhibition of SAHA-based non-hydroxamates. J. Med. Chem. 2005;48:1019–1032. Bouchain G., Delorme D. Novel hydroxamate and anilide derivatives as potent histone deacetylase inhibitors: synthesis and antiproliferative evaluation. Curr. Med. Chem. 2003;10:2359–2372. Shinji C., Maeda S., Imai K., Yoshida M., Hashimoto Y., Miyachi H. Design, synthesis, and evaluation of cyclic amide/imide-bearing hydroxamic acid derivatives as class-selective histone deacetylase (HDAC) inhibitors. Bioorg. Med. Chem. 2006;14:7625–7651. Kim H. M., Lee K., Park B. W., Ryu D. K., Kim K., Lee C. W., Park S. K., Han J. W., Lee H. Y., Lee H. Y., Han G. Synthesis, enzymatic inhibition, and cancer cell growth inhibition of novel δ-lactam-based histone deacetylase (HDAC) inhibitors. Bioorg. Med. Chem. Lett. 2006;16:4068–4070. Fournel M., Trachy–Bourget M. C., Yan P. T., Kalita A., Bonfils C., Beaulieu C., Frechette S., Leit S., Abou–Khalil E., Woo S. H., Delorme D., MacLeod A. R., Besterman J. M., Li Z. Sulfonamide anilides, a novel class of histone deacetylase inhibitors, are antiproliferative against human tumors. Cancer Res. 2002;62:4325–4330. Owa T., Yoshino H., Yoshimatsu K., Nagasu T. Cell cycle regulation in the G1 phase: a promising target for the development of new chemotherapeutic anticancer agents. Curr. Med. Chem. 2001;8:1487–1503. Bouchain G., Delorme D. Novel hydroxamate and anilide derivatives as potent histone deacetylase inhibitors: synthesis and antiproliferative evaluation. Curr. Med. Chem. 2003;10:2359–2372. Uesato S., Kitagawa M., Nagaoka Y., Maeda T., Kuwajima H., Yamori T. Novel histone deacetylase inhibitors: N-hydroxycarboxamides possessing a terminal bicyclic aryl group. Bioorg. Med. Chem. Lett. 2002;12:1347–1349. Lavoie R., Bouchain G., Frechette S., Woo S. H., Abou–Khalil E., Leit S., Fournel M., Yan P. T., Trachy–Bourget M. C., Beaulieu C., Li Z., Besterman J., Delorme D. Design and synthesis of a novel class of histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2001;11:2847–2850.
-
-
- Angibaud P., Arts J., Van Emelen K., Poncelet V., Pilatte I., Roux B., Van Brandt S., Verdonck M., De Winter H., Ten Holte P., Marien A., Floren W., Janssens B., Van Dun J., Aerts A., Van Gompel J., Gaurrand S., Queguiner L., Argoullon J. M., Van Hijfte L., Freyne E., Janicot M. Discovery of pyrimidyl-5-hydroxamic acids as new potent histone deacetylase inhibitors. Eur. J. Med. Chem. 2005;40:597–606. doi: 10.1016/j.ejmech.2005.01.008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources