Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression
- PMID: 17874037
- PMCID: PMC2384236
- DOI: 10.1007/s11060-007-9400-9
Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression
Abstract
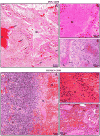
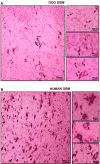
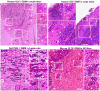
Although rodent glioblastoma (GBM) models have been used for over 30 years, the extent to which they recapitulate the characteristics encountered in human GBMs remains controversial. We studied the histopathological features of dog GBM and human xenograft GBM models in immune-deficient mice (U251 and U87 GBM in nude Balb/c), and syngeneic GBMs in immune-competent rodents (GL26 cells in C57BL/6 mice, CNS-1 cells in Lewis rats). All GBMs studied exhibited neovascularization, pleomorphism, vimentin immunoreactivity, and infiltration of T-cells and macrophages. All the tumors showed necrosis and hemorrhages, except the U87 human xenograft, in which the most salient feature was its profuse neovascularization. The tumors differed in the expression of astrocytic intermediate filaments: human and dog GBMs, as well as U251 xenografts expressed glial fibrillary acidic protein (GFAP) and vimentin, while the U87 xenograft and the syngeneic rodent GBMs were GFAP(-) and vimentin(+). Also, only dog GBMs exhibited endothelial proliferation, a key feature that was absent in the murine models. In all spontaneous and implanted GBMs we found histopathological features compatible with tumor invasion into the non-neoplastic brain parenchyma. Our data indicate that murine models of GBM appear to recapitulate several of the human GBM histopathological features and, considering their reproducibility and availability, they constitute a valuable in vivo system for preclinical studies. Importantly, our results indicate that dog GBM emerges as an attractive animal model for testing novel therapies in a spontaneous tumor in the context of a larger brain.
Figures



References
-
- Castro MG, Cowen R, Williamson IK, David A, Jimenez-Dalmaroni MJ, Yuan X, Bigliari A, Williams JC, Hu J, Lowenstein PR. Current and future strategies for the treatment of malignant brain tumors. Pharmacol Ther. 2003;98:71–108. - PubMed
-
- Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2:938–950. - PubMed
-
- Gomez-Manzano C, Yung WK, Alemany R, Fueyo J. Genetically modified adenoviruses against gliomas: from bench to bedside. Neurology. 2004;63:418–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS054193/NS/NINDS NIH HHS/United States
- 1 RO1 NS 054193.01/NS/NINDS NIH HHS/United States
- R21 NS047298/NS/NINDS NIH HHS/United States
- U54 NS045309/NS/NINDS NIH HHS/United States
- 1UO1 NS052465.01/NS/NINDS NIH HHS/United States
- F32 NS058156/NS/NINDS NIH HHS/United States
- 1R21-NS054143.01/NS/NINDS NIH HHS/United States
- R01 NS042893/NS/NINDS NIH HHS/United States
- R03 TW006273/TW/FIC NIH HHS/United States
- 1 RO3 TW006273-01/TW/FIC NIH HHS/United States
- R21 NS054143/NS/NINDS NIH HHS/United States
- 1F32 NS058156.01/NS/NINDS NIH HHS/United States
- NS445561/NS/NINDS NIH HHS/United States
- R01 NS044556/NS/NINDS NIH HHS/United States
- 1R01 NS44556.01/NS/NINDS NIH HHS/United States
- U01 NS052465/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

